A compound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass. Part A How many grams of C and H are there in a 190.0 g sample of this substance? Express your answers using one decimal place separated by a comma. 167.5,22.5 тс, тн = Previous Answers Submit Correct Part B How many moles of C and H? Express your wers using three significant figures separated by comma. 13.9,22.4 mol пс, пн — Previous Answers Submit Correct Part C What is the empirical formula? Express your answer as a chemical formula.
A compound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass. Part A How many grams of C and H are there in a 190.0 g sample of this substance? Express your answers using one decimal place separated by a comma. 167.5,22.5 тс, тн = Previous Answers Submit Correct Part B How many moles of C and H? Express your wers using three significant figures separated by comma. 13.9,22.4 mol пс, пн — Previous Answers Submit Correct Part C What is the empirical formula? Express your answer as a chemical formula.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 160IL: When analyzed, an unknown compound gave these experimental results: C, 54.0%; h, 6.00%; and O,...
Related questions
Question
100%
Given the answers to the previous questions, please answer part c (find the empirical formula)
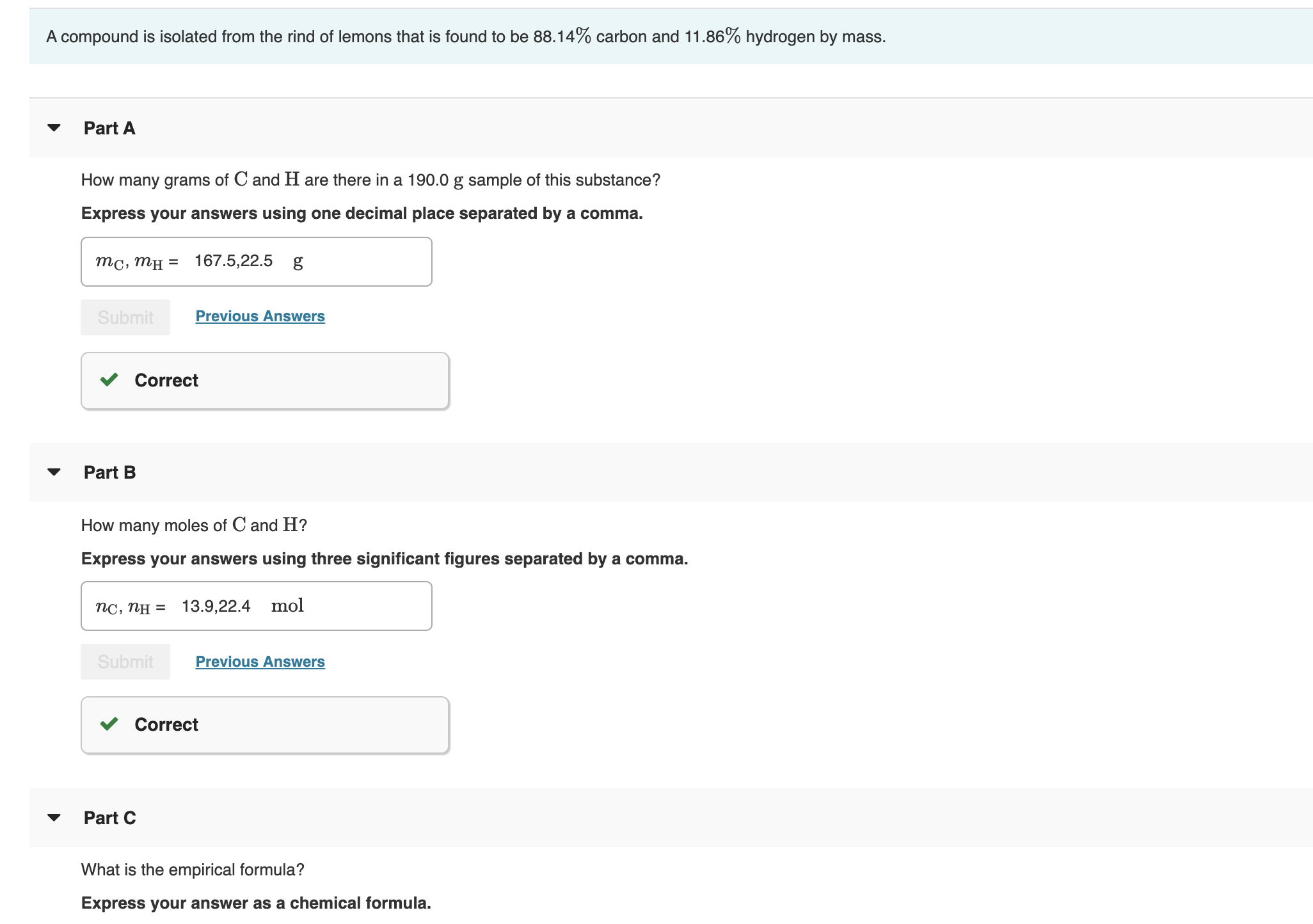
Transcribed Image Text:A compound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass.
Part A
How many grams of C and H are there in a 190.0 g sample of this substance?
Express your answers using one decimal place separated by a comma.
167.5,22.5
тс, тн
=
Previous Answers
Submit
Correct
Part B
How many moles of C and H?
Express your
wers using three significant figures separated by
comma.
13.9,22.4 mol
пс, пн —
Previous Answers
Submit
Correct
Part C
What is the empirical formula?
Express your answer as a chemical formula.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning