A crystal of sodium chloride ( in the absence of water) would contain bonds. O lonic O all of the above O Hydrogen O polar covalent O non-polar covalent Question 36 Which lipid would likely be a liquid at room temperature? Palmitic acid (a 16-carbon saturated fatty acid) O Oleic acid (an 18-carbon monounsaturated fatty acid) O Stearic acid (an 18-carbon saturated fatty acid) O Lauric acid (a 12-carbon saturated fatty acid)
A crystal of sodium chloride ( in the absence of water) would contain bonds. O lonic O all of the above O Hydrogen O polar covalent O non-polar covalent Question 36 Which lipid would likely be a liquid at room temperature? Palmitic acid (a 16-carbon saturated fatty acid) O Oleic acid (an 18-carbon monounsaturated fatty acid) O Stearic acid (an 18-carbon saturated fatty acid) O Lauric acid (a 12-carbon saturated fatty acid)
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question

Transcribed Image Text:1 pts
Enzymes alter the free energy change of a reaction.
True
O False
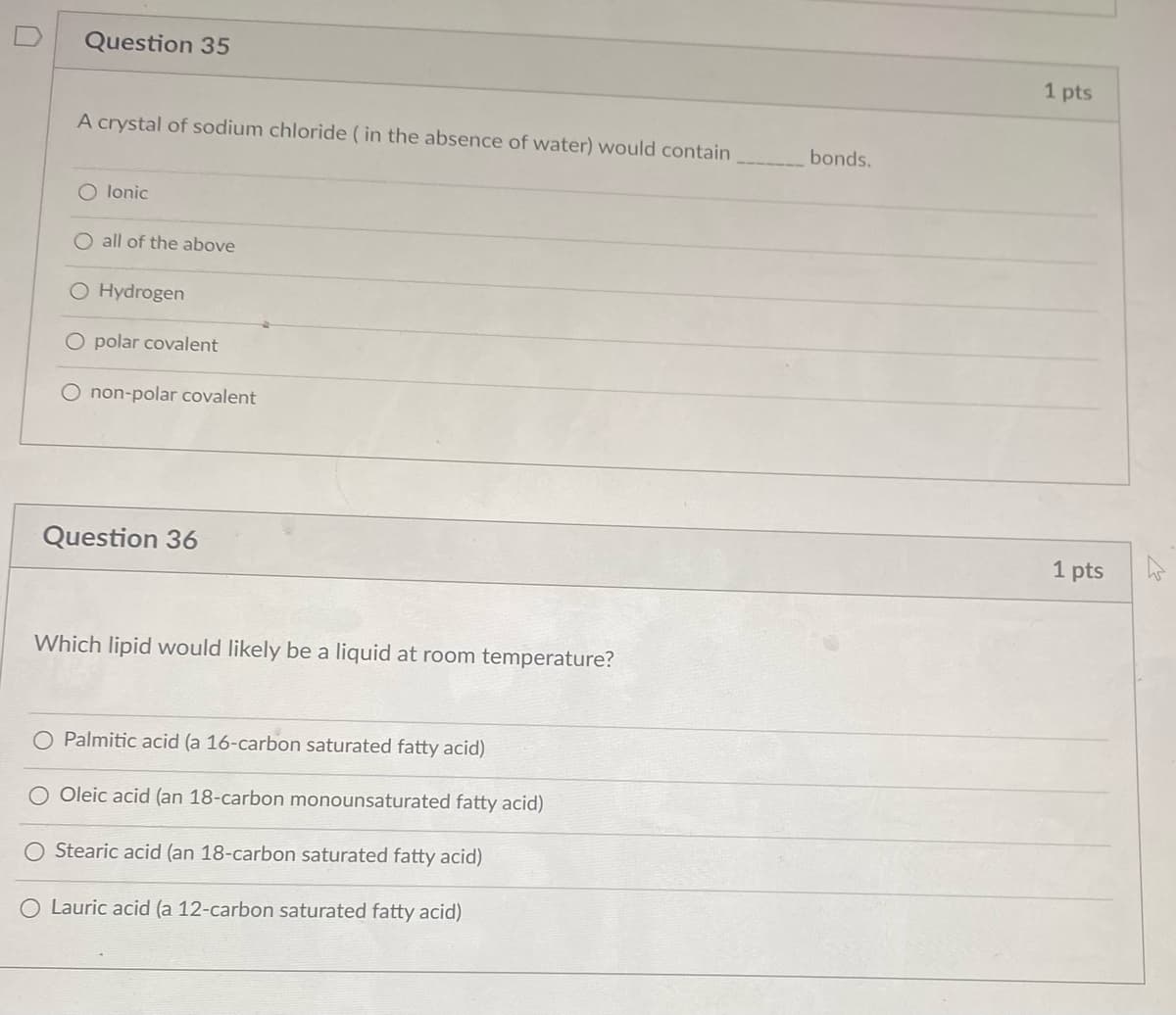
Transcribed Image Text:Question 35
1 pts
A crystal of sodium chloride ( in the absence of water) would contain
bonds.
O lonic
O all of the above
Hydrogen
O polar covalent
O non-polar covalent
Question 36
1 pts
Which lipid would likely be a liquid at room temperature?
O Palmitic acid (a 16-carbon saturated fatty acid)
Oleic acid (an 18-carbon monounsaturated fatty acid)
O Stearic acid (an 18-carbon saturated fatty acid)
O Lauric acid (a 12-carbon saturated fatty acid)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON