a Express the following measurement in scientific notation 2574.2 mL 2574.2 x 103 mL 2.5742 x 103 mL 2574.2 x 102 mL 2574.2 mL b Express the following measurement in scientific notation. 1.0001 g 1.0001 x 103 g 1.0001 x 10 g 1.0001 x 102 g 1.0001 g Submit C Express the following measurement in scientific notation. 100.70C 100.7 x 103C 100.7 x 103C 1 007 x 1020
a Express the following measurement in scientific notation 2574.2 mL 2574.2 x 103 mL 2.5742 x 103 mL 2574.2 x 102 mL 2574.2 mL b Express the following measurement in scientific notation. 1.0001 g 1.0001 x 103 g 1.0001 x 10 g 1.0001 x 102 g 1.0001 g Submit C Express the following measurement in scientific notation. 100.70C 100.7 x 103C 100.7 x 103C 1 007 x 1020
Chapter1: Matter, Measurements, And Calculations
Section: Chapter Questions
Problem 1.56E
Related questions
Question
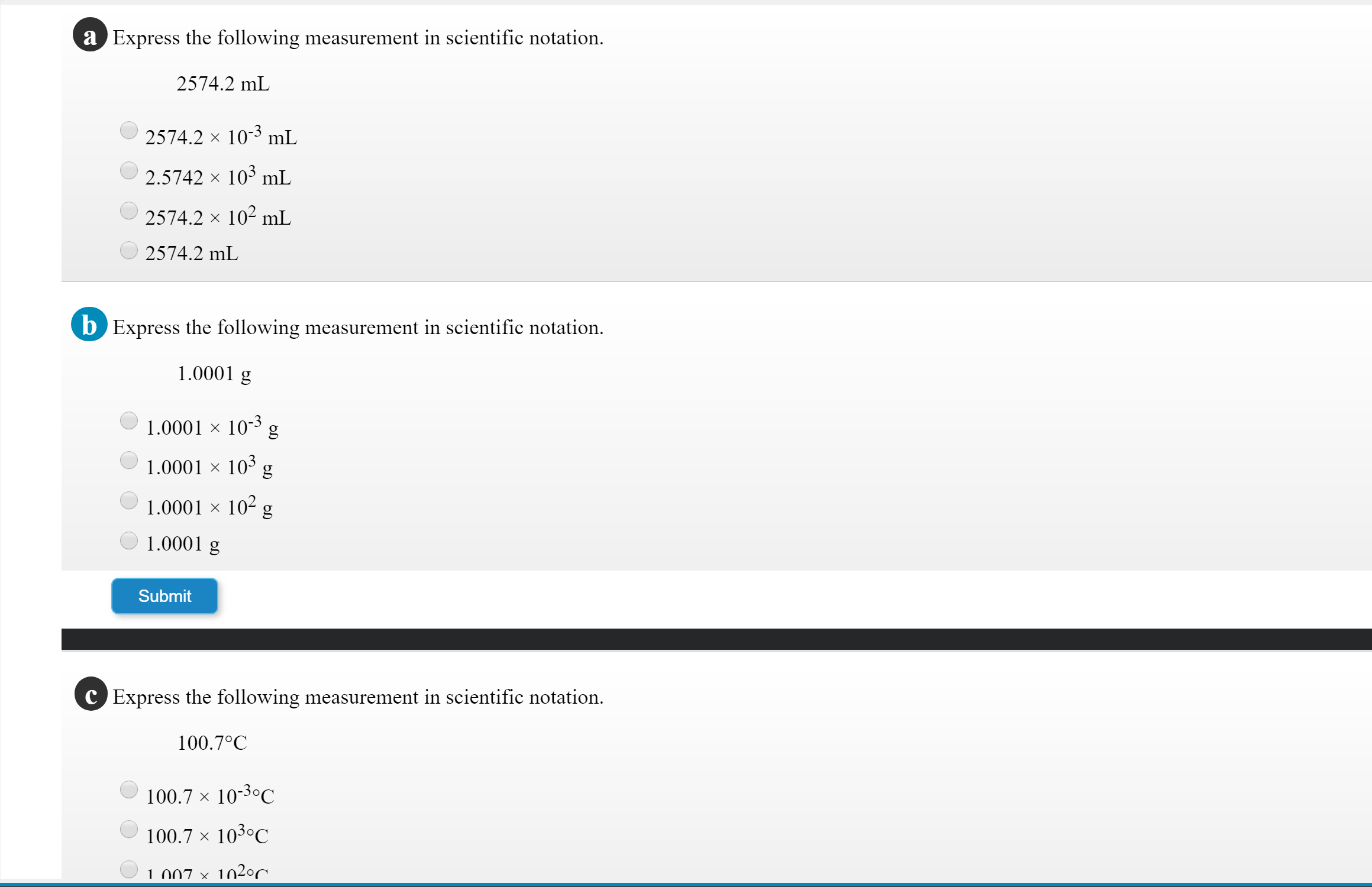
Transcribed Image Text:a Express the following measurement in scientific notation
2574.2 mL
2574.2 x 103 mL
2.5742 x 103 mL
2574.2 x 102 mL
2574.2 mL
b Express the following measurement in scientific notation.
1.0001 g
1.0001 x 103 g
1.0001 x 10 g
1.0001 x 102 g
1.0001 g
Submit
C Express the following measurement in scientific notation.
100.70C
100.7 x 103C
100.7 x 103C
1 007 x 1020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning