A galvanic (voltaic) cell contains a copper cathode immersed in a copper(II) chloride solution and a nickel anode immersed in a nickel(II) chloride solution. The two solutions are connected with a salt bridge. Write the balanced equation for the galvanic cell. Phases are optional. balanced equation: A current of 2.40 A is observed flowing through the cell for a period of 2.81 h. Calculate the amount of charge flowing through the circuit during this time. Calculate the number of moles of electrons flowing through the circuit during this time. moles of clectrons: Calculate the mass change in grams of the copper and nickel electrodes. mass change of copper electrode: mass change of nickel electrode:
A galvanic (voltaic) cell contains a copper cathode immersed in a copper(II) chloride solution and a nickel anode immersed in a nickel(II) chloride solution. The two solutions are connected with a salt bridge. Write the balanced equation for the galvanic cell. Phases are optional. balanced equation: A current of 2.40 A is observed flowing through the cell for a period of 2.81 h. Calculate the amount of charge flowing through the circuit during this time. Calculate the number of moles of electrons flowing through the circuit during this time. moles of clectrons: Calculate the mass change in grams of the copper and nickel electrodes. mass change of copper electrode: mass change of nickel electrode:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 97AP
Related questions
Question
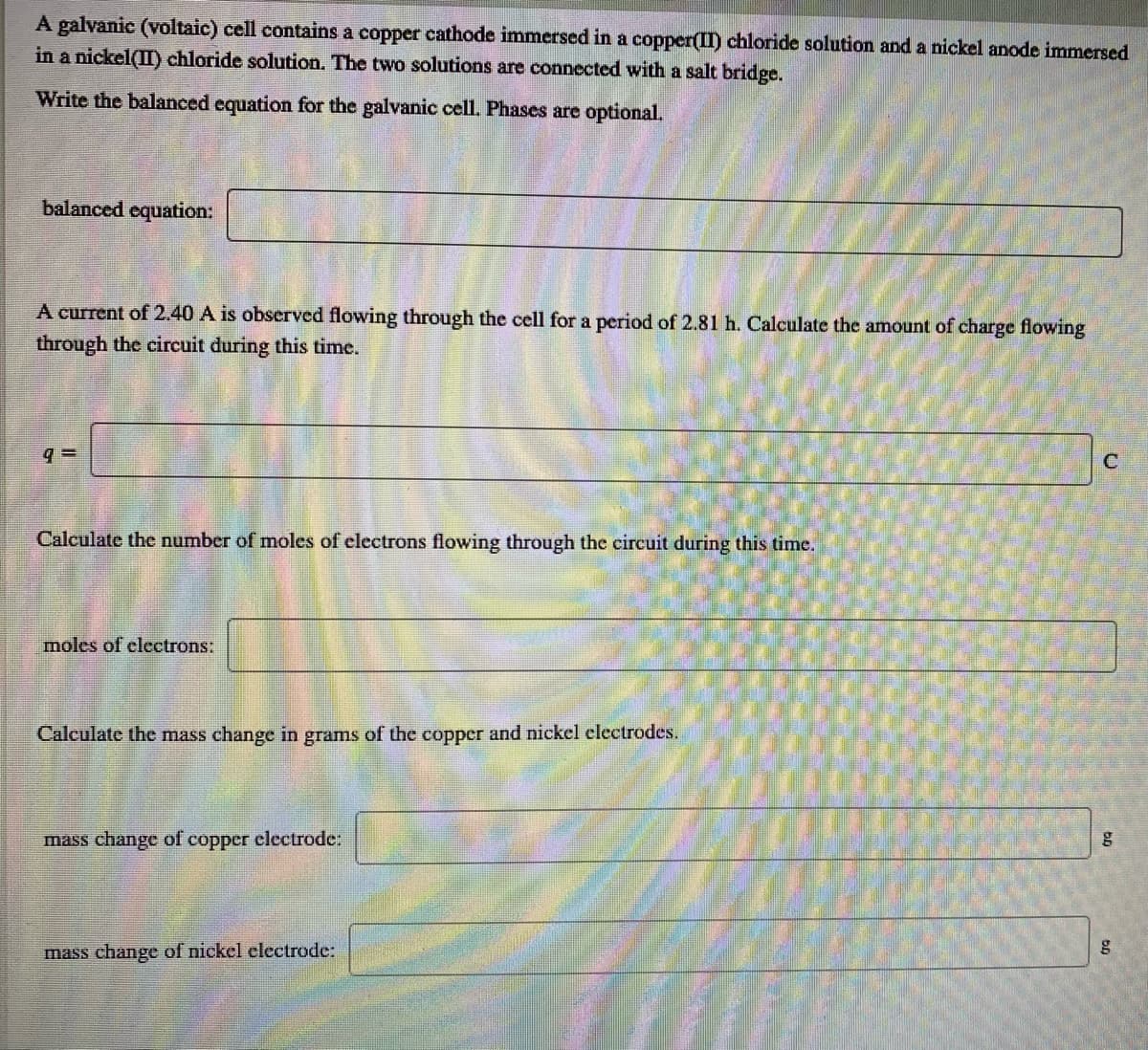
Transcribed Image Text:A galvanic (voltaic) cell contains a copper cathode immersed in a copper(I) chloride solution and a nickel anode immersed
in a nickel(II) chloride solution. The two solutions are connected with a salt bridge.
Write the balanced equation for the galvanic cell. Phases are optional.
balanced equation:
A current of 2.40 A is observed flowing through the cell for a period of 2.81 h. Calculate the amount of charge flowing
through the circuit during this time.
C
Calculate the number of moles of electrons flowing through the circuit during this time.
moles of clectrons:
Calculate the mass change in grams of the copper and nickel electrodes.
mass change of
copper clectrodc:
mass change of nickel electrode:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning