A liquid has its vapor pressure measured at several temperatures. A graph of the In P vs 1/T is prepared and appears below. If the equation for the line is.. y = -4699.3 k X + 2.38 What is AH for the liquid in kJ/mol? 'vap Your answer should have 4 sig. figs. Do not include units on the typed answer. Just type in the number. If you want a number in scientific notation such as 0.00034, type 3.4e-4. Inp 1/T Answer:
A liquid has its vapor pressure measured at several temperatures. A graph of the In P vs 1/T is prepared and appears below. If the equation for the line is.. y = -4699.3 k X + 2.38 What is AH for the liquid in kJ/mol? 'vap Your answer should have 4 sig. figs. Do not include units on the typed answer. Just type in the number. If you want a number in scientific notation such as 0.00034, type 3.4e-4. Inp 1/T Answer:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 13QAP
Related questions
Question
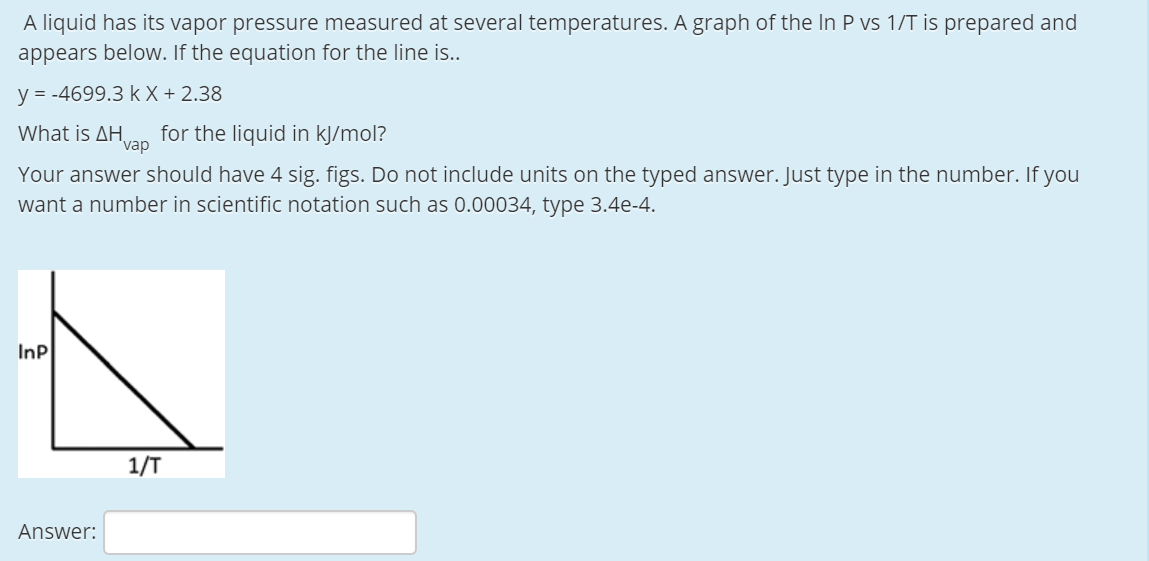
Transcribed Image Text:A liquid has its vapor pressure measured at several temperatures. A graph of the In P vs 1/T is prepared and
appears below. If the equation for the line is..
y = -4699.3 k X + 2.38
What is AH
for the liquid in kJ/mol?
'vap
Your answer should have 4 sig. figs. Do not include units on the typed answer. Just type in the number. If you
want a number in scientific notation such as 0.00034, type 3.4e-4.
Inp
1/T
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,