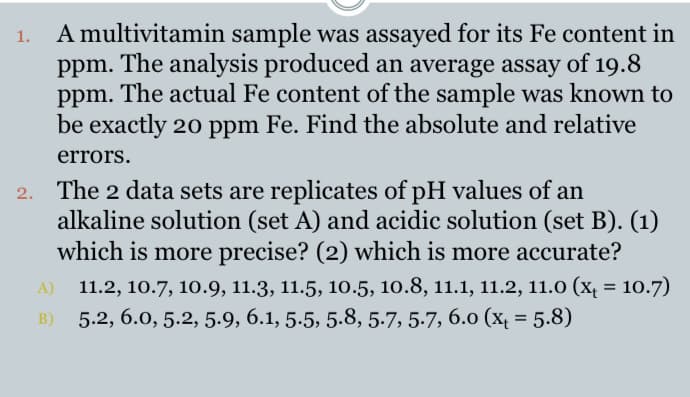
A multivitamin sample was assayed for its Fe content in ppm. The analysis produced an average assay of 19.8 ppm. The actual Fe content of the sample was known to be exactly 20 ppm Fe. Find the absolute and relative 1. errors.
Q: How many of the following measures are quantitative?
A: A variable is said to be quantitative if it can be measured numerically and operations like…
Q: A sample of n = 4 scores has a mean of x̅ = 40, with a sum of squares of SS = 48. What is the…
A: Given that n=4, x̅ = 40 and SS=48 Consider, the variance, s2=SSn-1=484-1=16
Q: Determine the largest relative error in a calculation of the cross-sectional area of a wire from a…
A: The relative error is calculated by subtracting the expected value from the actual value and…
Q: I found my critical value t is 2.756, s= 0.64, and n=30 what is the margin of error
A: given; critical value of t, t*=2.756 standard deviation(s)=0.64 sample size(n)=30 find margin of…
Q: find the the z score shaded area for given data (-3.21<z<2.2587)
A: Given probability is P(-3.21<z<2.2587)
Q: A researcher reports an F-ratio with df=3,70df=3,70 for an independent-measures research study.…
A: Given: A researcher reports an F-ratio with df=3,70
Q: WUmens satisfaction with natural deodorants. (Round to the nearest whole person as needed). Is there…
A:
Q: Ex. X = 400 Y = 700 Exr = 15, 000 Σε X2 = 16, 229 y2 = 50, 000
A:
Q: chi-square test for goodness of fit has df = 4. How many categories were used to classify the…
A: Given that ; chi-square test for goodness of fit has df = 4. By using the concept of chi- square…
Q: . If the average value of f (r) is 16, find the value of f(r) dr
A:
Q: The results for a blood test for a certain disease are shown in the tab below. Let D represent…
A: here True positive = 290 False negative = 3219
Q: If the the true value = 11.5140 and the true Error = -0.1822 then the Relative True Percentage…
A: This is a problem of Numerical Analysis.
Q: In a class of 50 students, Heather has a rank of 14. At what percentile is she? Heather has a…
A: According to the provided information, B = 14 N = 50
Q: A researcher reports an F-ratio with df= 2, 18 from an independent-measures research study. Based on…
A: It is an important part of statistics . It is widely used .
Q: a) If a speedometer's absolute error is 2 mph and it measures a speed of 60 mph, what is the…
A: Note: Hi! Thank you for the question as per the honor code, we’ll answer the first question since…
Q: What is the uncertainty or Standard Error for this difference in proportions? (F
A: Here For People uses Internet Regularly Sample Size=n1=1754 x1=807 p1^=8071754=0.46 Now For people…
Q: Compute the relative error if the true or actual value is 45.84 cm and the measured value is 46.00…
A:
Q: A researcher reports an F-ratio with df = 3, 36 from an independent-measures research study. How…
A: A researcher reports an F-ratio with df = 3, 36 from an independent-measures research study. It is…
Q: A researcher reports an F-ratio with df=2,27 from an independent-measures research study. How many…
A: Given, A researcher reports an F-ratio with df=2,27 from an independent-measures research study. Let…
Q: - If the uncertainty for X is 0.4 mm. How many measurements are needed so that the uncertainty…
A: Hi! Thank you for posting the question. Since you have posted multiple questions, we are answering…
Q: Use the linear approximation to estimate (-3.01)*(1.98)² ~ Compare with the value given by a…
A: given that (-3.01)3(1.98)2 let function be…
Q: What is the value of SS Between?
A: The data shows the ratings of five different engineering board examinations.
Q: If comparing two measurements of different quantities, the true error is sufficient in deducing the…
A:
Q: The average value of y = v(x) equals 8 for 1< x < 6, and equals 9 for 6 < x < 8. What is the average…
A:
Q: S For n= 15, the margin of error for an estimate at an alpha level of 2% is +2.249 15
A:
Q: The average value of y=v(x) is 4 for 2≤x≤5 and is 10 for 5≤x≤12. What is the average value of v(x)…
A: Given, The average value of y=v(x) is 4 for 2 ≤ x ≤ 5 The average value of y=v(x) is 10…
Q: Compare the two groups (male and female students) in terms of their measures of location and…
A: Introduction: To compare the two groups, we have first separately identified the students in the two…
Q: The data show the particulate matter in micrograms per cubic meter for a sample of large cities on…
A: The null and alternative hypothesis is as follows: Null hypothesis: H0:µ1 = µ2 = µ3 Alternative…
Q: Suppose the relationship between Y and X is given by: Y = 10 + 8.75*X + error By how much does…
A: Solution: From the given information, Y=10+8.75*X+ error
Q: The z score length of a carrot is 1.5, what is the length of this carrot? what percentage of…
A: We have to find the percentage of carrots....
Q: For paired observations, the value of the average difference is?
A: Detaild Explanation Given In Attached File kindly Refer.
Q: If your study consists of four groups, each with 15 subjects, and SSpet = 42 and SSw = 35, what does…
A: Number of groups, k = 4 Number of observations, N = 60 SSbet = 42 and SSw = 35
Q: A researcher reports an F-ratio with df=4,98 for an independent-measures research study. How many…
A: A researcher reports an F-ratio with df=4,98 for an independent-measures research study.
Q: The manager of an orchard expects about 82 percent of her apples to exceed the weight requirements…
A:
Q: What is the measure of YZ?
A:
Q: A student at Tolland High School wondered if boys or girls at her school traveled a greater distance…
A: Given: Sample size for girls = 50 Sample size for boys = 50 The concern is to check the average…
Q: Is IQ related to GPA?If a student’s IQ is 117, what GPA would you predict?How much of the variation…
A: Given Info : The provided data are from a random sample of thirty cases from a study by Howell…
Q: The coefficient of variation for the systolic measurements is ___ %? The coefficient of variation…
A: Solution: The systolic and Diastolic measurements for a group of 10 adults are given. The…
Q: 1. If a speedometer's absolute error is 1 mph and it measures a speed of 55 mph, what is the…
A:
Q: Can pleasant aromas help a student learn better? Hirsch and Johnston, of the Smell & Taste Treatment…
A: Data are given, a) Here we used paired t-test because here we checking effects of wearing a…
Q: Does the t test indicate a significant relationship between price and the overall score? Compute…
A: Note: Since you have mentioned (a) subdivision separately we will answer the same. a) In the given…
Q: 1. Clearly indicate why a paired t-test should be used in this analysis. Then produce the…
A: 1. The paired t test is used when the subjects in the study are measured twice, that is the same…
Q: In a class of 80 students, Heather has a rank of 16. At what percentile is she? Heather has a…
A: Heather has rank 16 in a class of 80 students. The number of students having rank after Heather is,…
Q: Because of the difficulty of weighing a Bear in the woods, researchers caught and measured 20 Bear,…
A: Quantitative variables take only numerical values.
Q: When X; only takes two values {0, 1}, what are the possible values of the estimates of p?
A: Solution : Given that , X1,X2,...........Xn be a random sample from population.Considering…
Q: When estimating the population proportion, how large a sample is needed to be 95% confident that the…
A: Here sample proportion = 42/120= 0.35
Q: Use the empirical rule to choose the best value for the percentage of the area under the curve that…
A: here given mean = 41.8 cm and standard deviation = 5.3 cm
Q: Scores on an accounting exam ranged from 42 to 92, with quartiles Q1= 59.00, Q2= 79.0, and Q3=82.25.…
A: (a) Procedure for constructing boxplot: The x-axis represents the units of data values ranging from…
Q: Use the linear approximation to estimate (-3,01)*(1.98)² = Compare with the value given by a…
A: From the given question, we can state that: f(x,y)=x3y2. We know that: Linear approximation of…
Step by step
Solved in 2 steps

- Q1 A) List down the measures of central tendency and measures of dispersion 2) The operations manager of a plant that manufactures tires wants to compare the actual inner diameters of two grades of tires, each of B) which is expected to be 575 millimeters. A sample of five tires of each grade was selected, and the results representing the inner diameters of the tires, ranked from smallest to largest, are as follows. Grade X grade Y 568 570 575 578 584 573 574 575 577 578 requirement. a) for each of the tow grades of tries, compute the mwan, median, and standred deviation. b) which grade of tire providing better quality? explain. c) what would be the effect on your answer in (a) and (b) if the last value for grade Y were 588 insert 578 explain. C) The file contins the overall miles per gallon (MPG) OF 2010 family sedan: 24 21 22 23 24 34 34 34 20 20 22 22 44 32 20 20 22 20 39 20 Source:…Penicillin is produced by the Penicillin fungus, which is grown in a broth whose sugar content must be carefully controlled. Several samples of broth were taken on three successive days, and the amount of dissolved sugars, in milligrams per milliliter, was measured on each sample. The results were as follows. Day 1 : 4.9 5.4 5.3 4.9 5.2 5.1 5.4 4.9 5.1 5.1 4.9 5.4 Day 2 : 5.5 5.2 5.1 5.0 5.3 5.4 5.3 5.2 5.4 5.3 5.4 5.1 Day 3 : 5.8 5.0 5.4 5.5 5.5 5.5 4.8 5.5 5.2 4.9 5.5 5.0 Construct an ANOVA table. Round your answers to four decimal places as needed. One-way ANOVA: Sugar Concentration Source DF SS MS F P Days Error Total Is there enough evidence to conclude that the mean sugar concentration…If Elliot collects data from a single sample and her dependent variable is assessed on a nominal scale, which of these difference tests would Elliot need to use to analyze her data? a. single sample t test b. between-subjects, one-way ANOVA. c. chi square goodness of fit test d. single-sample z test
- The performance of two analysts in determining total mercury (mg / kg) in fish was evaluated using the absorption spectrometry method. The results on the concentrations of mercury found in fish, for each analyst, are presented in the table below: Analyst 1 Analyst 2 7 12 9 8 6 9 11 13 13 14 8 9 7 8 13 10 12 7 9 15 (a)Is the performance in determining total mercury for Analyst 1 more accurate than Analyst 2? Compare the accuracy in the results of the two analysts at a 95% confidence level. Explain. (b)Are the results obtained by the two analysts, in the concentrations of mercury found in the fish, statistically equivalent, with 95% confidence? ExplainAs a bonus assignment a former student checked if your professor gave a statisticallysignificant difference in grades between his male and female students. She based herstudy based on grades assigned in intermediate Econ courses (Econ 303, 305 and 317)and her sample included nm = 485 male students and nf = 264 female students. Theaverage grades received were xm = 84.6 and xf = 85.8 The population standad deviation were σ m = 12.0 and σ f = 11.4 8. From the same extra-credit study as in question 7 see above, this former student found that the proportion of female students in principle courses (Econ 203, 205) was ?̅? = 0.380, while the proportion of female students in intermediate courses (Econ 303, 305, 317) was ?̅? = 0.352. The principle courses sample size was np = 782, while the intermediate courses sample size was ni = 749. Test the hypothesis that female students are less in intermediate courses using a 90% confidence level and the p-value approach.The Senator of Azenator State, is worried about the rising numbers in high blood pressure related deaths in his Jurisdiction and wants an end to this canker. A reputable medical research officer has claimed that, the situation is probably as a result of the ageing population of his State. The blood pressures, Y (mmHg), and Ages, X (years) of 10 hospital patients were sampled from Azenator State and summarized below. Patient A B C D E F G H I J Age(X) in Years 20 25 50 30 45 60 10 15 35 70 BP(Y) in (mmHg) 80 85 125 90 100 135 80 70 100 140 NB: Approximate to 2 decimal placesUse the table to answer the questions that follow;i) Calculate the product moment correlation coefficient for the data and interpret your result.ii) If the Senator decides to purchase and distribute Norvasc (a medicine that reduces blood pressure), based on your results in (i), which age group (youth or old adults) should be given priority? Briefly explain your answer. iii) Give a reason to support fitting…

