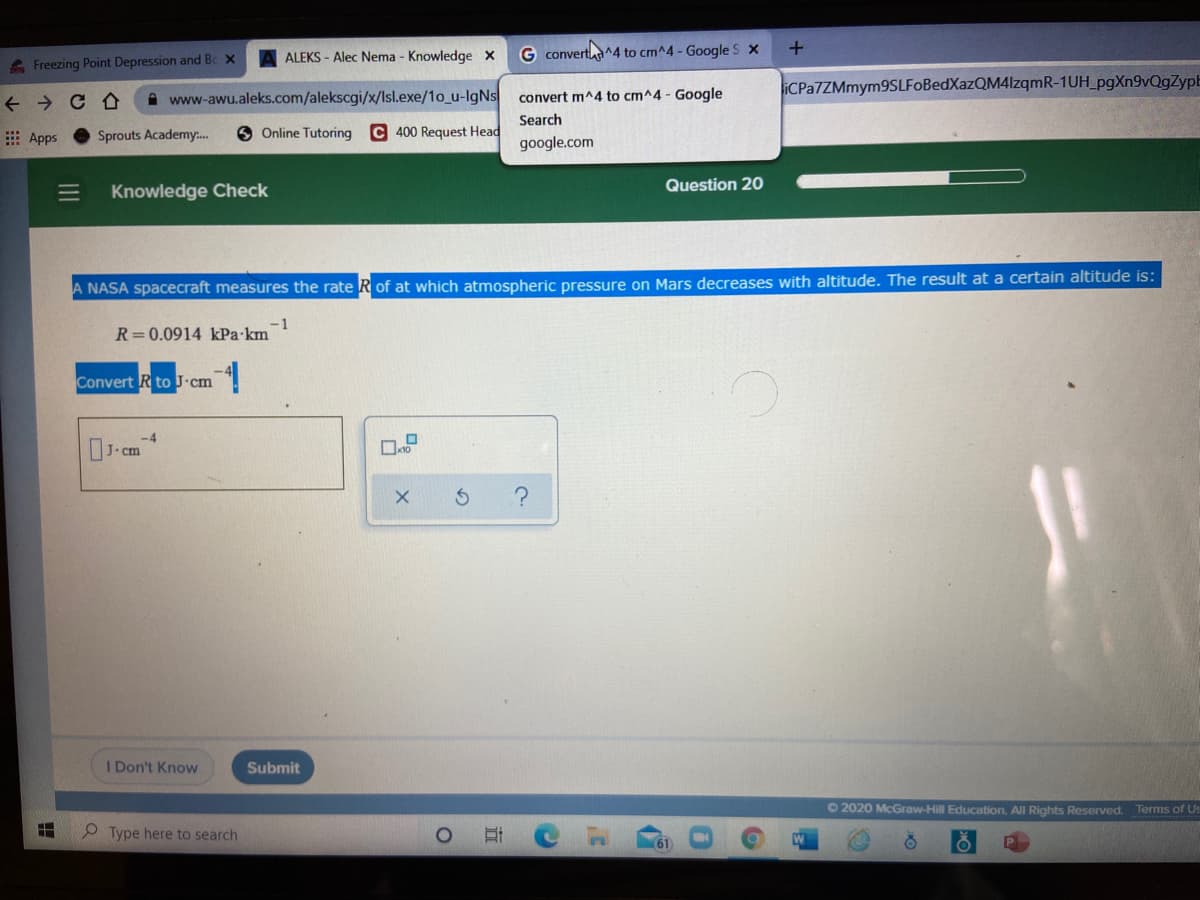
A NASA spacecraft measures the rate R of at which atmospheric pressure on Mars decreases with altitude. The result at a certain altitude is: -1 R=0.0914 kPa km Convert R to J•cm
A NASA spacecraft measures the rate R of at which atmospheric pressure on Mars decreases with altitude. The result at a certain altitude is: -1 R=0.0914 kPa km Convert R to J•cm
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 7E: 7.The word pour is commonly used in reference to liquids but not to solids or gases. Can you pour a...
Related questions
Question
100%
Transcribed Image Text:ALEKS - Alec Nema - Knowledge x
G converta^4 to cm^4- Google S x
AFreezing Point Depression and Bc X
iCPa7ZMmym9SLFOBedXazQM4lzqmR-1UH_pgXn9vQgZypE
-→ C合
www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNs
convert m^4 to cm^4- Google
Search
出 Apps
Sprouts Academy.
6 Online Tutoring C 400 Request Head
google.com
Question 20
Knowledge Check
A NASA spacecraft measures the rate R of at which atmospheric pressure on Mars decreases with altitude. The result at a certain altitude is:
1
R=0.0914 kPa•km
Convert Rto J.cm
-4
J cm
I Don't Know
Submit
O2020 McGraw-Hill Education, All Rights Reserved. Terms of Us
Type here to search
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning