A person tries to heat up her bath water by adding 5.0 L of water at 80 °C to 60 L of water at 30°C. What is the final temperature of the water? A) 38°C B) 36°C C) 40°C D) 34°C
A person tries to heat up her bath water by adding 5.0 L of water at 80 °C to 60 L of water at 30°C. What is the final temperature of the water? A) 38°C B) 36°C C) 40°C D) 34°C
College Physics
11th Edition
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter11: Energy In Thermal Processes
Section: Chapter Questions
Problem 18P: Lead pellets, each of mass 1.00 g, are heated to 200.C. How many pellets must be added to 0.500 kg...
Related questions
Question
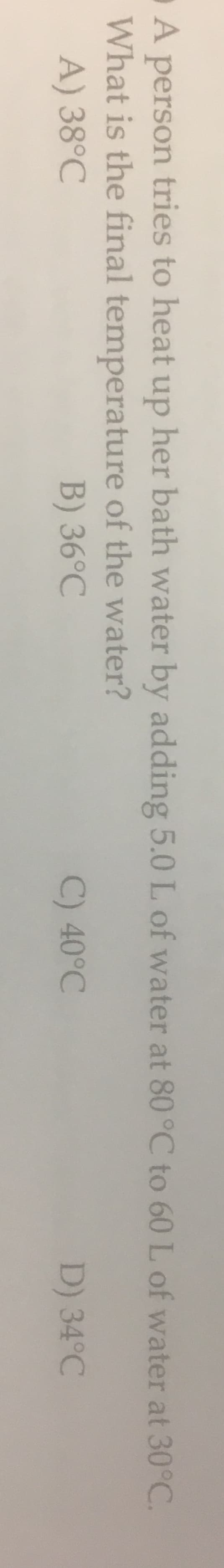
Transcribed Image Text:A person tries to heat up her bath water by adding 5.0 L of water at 80 °C to 60 L of water at 30°C.
What is the final temperature of the water?
A) 38°C
B) 36°C
C) 40°C
D) 34°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning