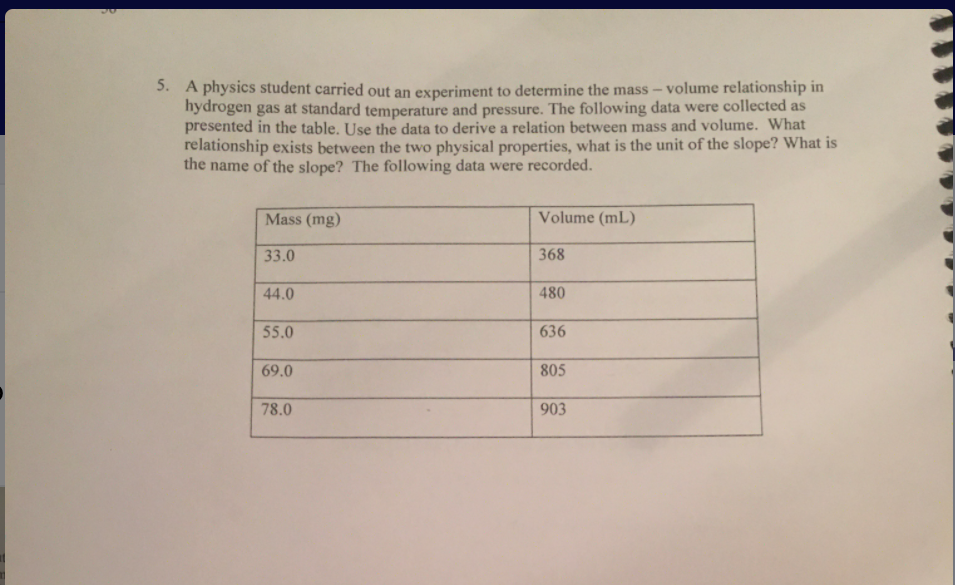
A physics student carried out an experiment to determine the mass – volume relationship in hydrogen gas at standard temperature and pressure. The following data were collected as presented in the table. Use the data to derive a relation between mass and volume. What relationship exists between the two physical properties, what is the unit of the slope? What is the name of the slope? The following data were recorded. Mass (mg) Volume (mL) 33.0 368 44.0 480 55.0 636 69.0 805 78.0 903
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images









