A piece of unknown substance weighs 55.8 g and requires 1910 J to increase its temperature from 21.7°C to 87.4°C. (a) What is the specific heat (in J/g-°C) of the substance? 4.0 J/g.°C (b) If it is one of the substances found in the table below, what is its likely identity? Specific Heats of Common Substances at 25°C and 1 bar Substance Symbol (state) Specific Heat (J/g-°C) O gold Au(s) 0.129 O copper Cu(s) 0.384 O iron Fe(s) 0.449 O argon Ar(g) 0.521 O silicon Si(s) 0.712 O carbon dioxide Co,(0) 0.843 O aluminum Al(s) 0.904 O nitrogen N2(g) 1.039 water H20(1) 4.1801 O helium He(g) 5.196 Type here to search hp 112 fs f6 144 sc & 8 Q E A J K B. FL %24
A piece of unknown substance weighs 55.8 g and requires 1910 J to increase its temperature from 21.7°C to 87.4°C. (a) What is the specific heat (in J/g-°C) of the substance? 4.0 J/g.°C (b) If it is one of the substances found in the table below, what is its likely identity? Specific Heats of Common Substances at 25°C and 1 bar Substance Symbol (state) Specific Heat (J/g-°C) O gold Au(s) 0.129 O copper Cu(s) 0.384 O iron Fe(s) 0.449 O argon Ar(g) 0.521 O silicon Si(s) 0.712 O carbon dioxide Co,(0) 0.843 O aluminum Al(s) 0.904 O nitrogen N2(g) 1.039 water H20(1) 4.1801 O helium He(g) 5.196 Type here to search hp 112 fs f6 144 sc & 8 Q E A J K B. FL %24
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 10E: A piece of unknown substance weighs 44.7 g and requires 2110 J to increase its temperature from 23.2...
Related questions
Question
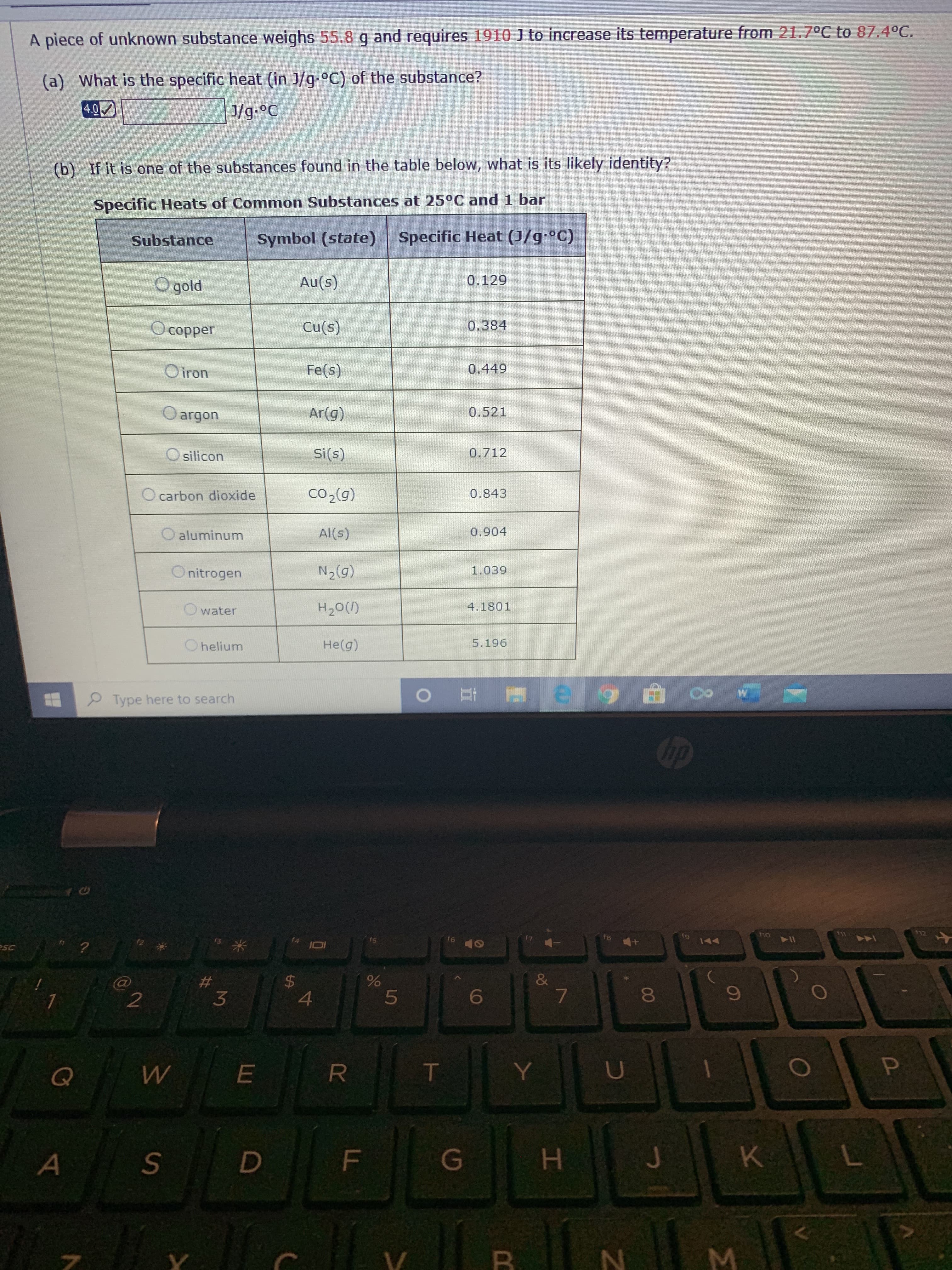
Transcribed Image Text:A piece of unknown substance weighs 55.8 g and requires 1910 J to increase its temperature from 21.7°C to 87.4°C.
(a) What is the specific heat (in J/g-°C) of the substance?
4.0
J/g.°C
(b) If it is one of the substances found in the table below, what is its likely identity?
Specific Heats of Common Substances at 25°C and 1 bar
Substance
Symbol (state) Specific Heat (J/g-°C)
O gold
Au(s)
0.129
O copper
Cu(s)
0.384
O iron
Fe(s)
0.449
O argon
Ar(g)
0.521
O silicon
Si(s)
0.712
O carbon dioxide
Co,(0)
0.843
O aluminum
Al(s)
0.904
O nitrogen
N2(g)
1.039
water
H20(1)
4.1801
O helium
He(g)
5.196
Type here to search
hp
112
fs
f6
144
sc
&
8
Q
E
A
J
K
B.
FL
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning