A piston-cylinder assembly contains a mystery substance that undergoes a series of processes. Process 1->2: Constant pressure process at 5bar from v1=.07m3/kg to v2=.12m3/kg Process 2->3: Constant volume process to saturated vapor Process 3->4: Constant temperature process to quality of 50% Process 4->5: Constant volume process to 5bar Sketch the processes on Pt, Pv, and Tv, plots. Lable the axes including property values; use closed dots to show the states, use solid lines to connect the states, add number and arrows to make clear the states numbers and process directions. Could this be considered a thermodynamic cycle? Why or why not?
A piston-cylinder assembly contains a mystery substance that undergoes a series of processes. Process 1->2: Constant pressure process at 5bar from v1=.07m3/kg to v2=.12m3/kg Process 2->3: Constant volume process to saturated vapor Process 3->4: Constant temperature process to quality of 50% Process 4->5: Constant volume process to 5bar Sketch the processes on Pt, Pv, and Tv, plots. Lable the axes including property values; use closed dots to show the states, use solid lines to connect the states, add number and arrows to make clear the states numbers and process directions. Could this be considered a thermodynamic cycle? Why or why not?
Automotive Technology: A Systems Approach (MindTap Course List)
6th Edition
ISBN:9781133612315
Author:Jack Erjavec, Rob Thompson
Publisher:Jack Erjavec, Rob Thompson
Chapter3: Basic Theories And Math
Section: Chapter Questions
Problem 2RQ: In what four states does matter exist? Cite examples of each.
Related questions
Question
100%
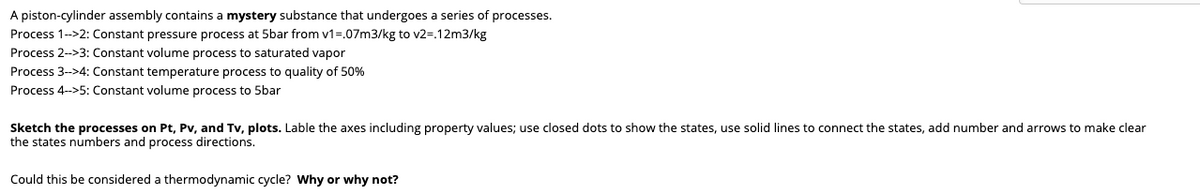
Transcribed Image Text:A piston-cylinder assembly contains a mystery substance that undergoes a series of processes.
Process 1-->2: Constant pressure process at 5bar from v1=.07m3/kg to v2=.12m3/kg
Process 2->3: Constant volume process to saturated vapor
Process 3->4: Constant temperature process to quality of 50%
Process 4->5: Constant volume process to 5bar
Sketch the processes on Pt, Pv, and Tv, plots. Lable the axes including property values; use closed dots to show the states, use solid lines to connect the states, add number and arrows to make clear
the states numbers and process directions.
Could this be considered a thermodynamic cycle? Why or why not?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning