A reaction between substances A and B has been found to give the following data: 3A +2B 2C+D Al (mol/L) (mol/Rate of appearance of C 10 x 102 10 x 102 30 2.0 x 102 30 mol / L-hr) 0.300 x 100 810x 10 3 24 x 10-5 1.20 x 10-8 30 x 10-5 10 0 x 102 1.0 3.0 x 10- Using the above data, determine: a) the order of the reaction with respect to A and B - b) the rate law, and - c) calculate the specific rate constant
A reaction between substances A and B has been found to give the following data: 3A +2B 2C+D Al (mol/L) (mol/Rate of appearance of C 10 x 102 10 x 102 30 2.0 x 102 30 mol / L-hr) 0.300 x 100 810x 10 3 24 x 10-5 1.20 x 10-8 30 x 10-5 10 0 x 102 1.0 3.0 x 10- Using the above data, determine: a) the order of the reaction with respect to A and B - b) the rate law, and - c) calculate the specific rate constant
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.35PAE: 11.35 For the reaction 2 NO(g) + 2 H?(g) — N,(g) + 2 H,O(g) at 1100°C, the following data have been...
Related questions
Question
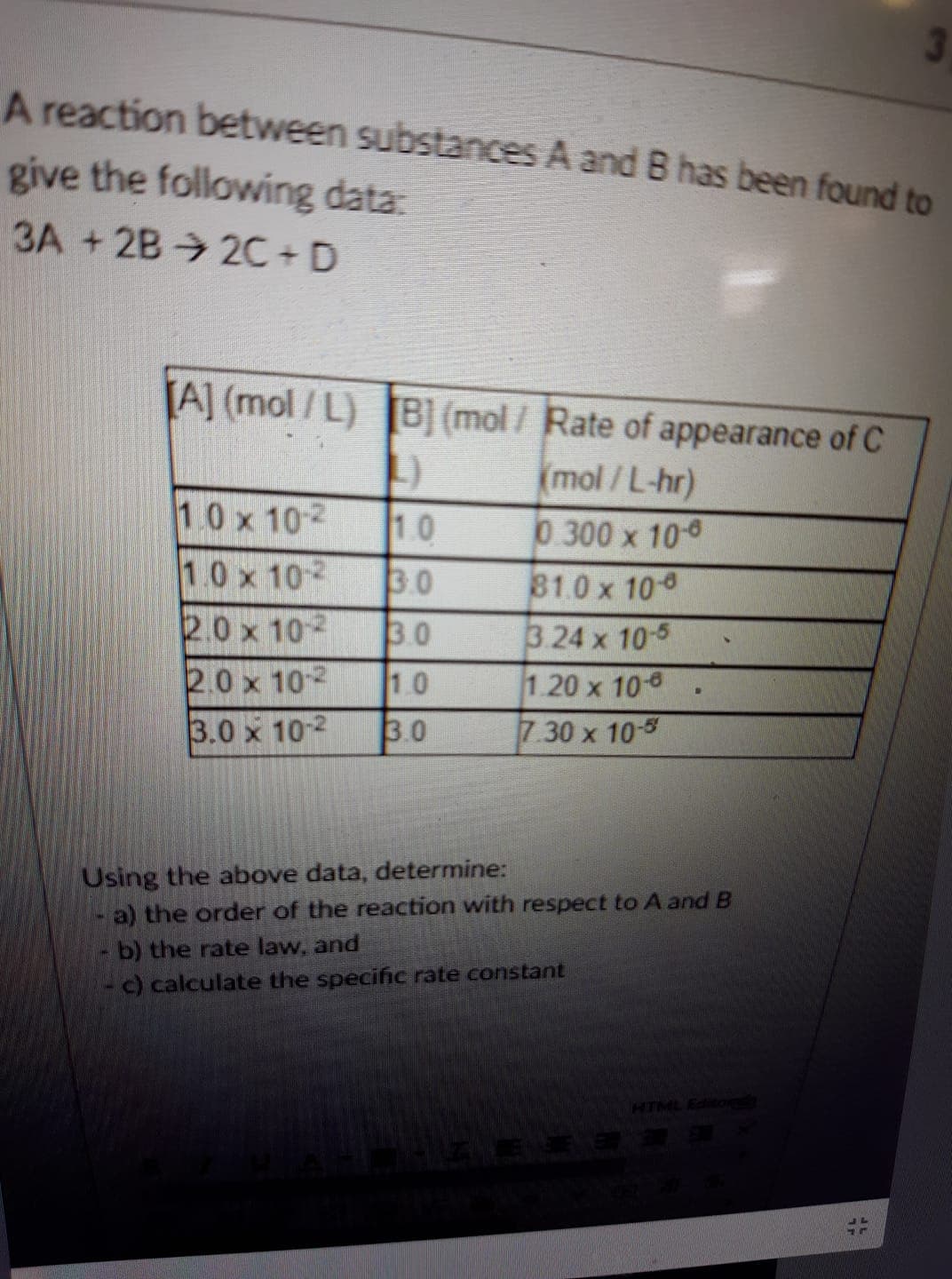
Transcribed Image Text:A reaction between substances A and B has been found to
give the following data:
3A +2B 2C+D
Al (mol/L)
(mol/Rate of appearance of C
10 x 102
10 x 102 30
2.0 x 102 30
mol / L-hr)
0.300 x 100
810x 10
3 24 x 10-5
1.20 x 10-8
30 x 10-5
10
0 x 102 1.0
3.0 x 10-
Using the above data, determine:
a) the order of the reaction with respect to A and B
- b) the rate law, and
- c) calculate the specific rate constant
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning