A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions Reaction Coordinate 1. Identify the transition state(s): 2. Which is the fastest reaction? Formation of C from A В Formation of E from C Formation of A from C D O Formation of C from E 3. Identify the rate limiting step 4. Which compound is the most unstable? A to form C OC to form E E to form C C to form A OooOo OO000 Free Energy
A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions Reaction Coordinate 1. Identify the transition state(s): 2. Which is the fastest reaction? Formation of C from A В Formation of E from C Formation of A from C D O Formation of C from E 3. Identify the rate limiting step 4. Which compound is the most unstable? A to form C OC to form E E to form C C to form A OooOo OO000 Free Energy
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section: Chapter Questions
Problem 4.22P: In each of the following three reaction coordinate diagrams, state: (a) Whether the reaction is...
Related questions
Question
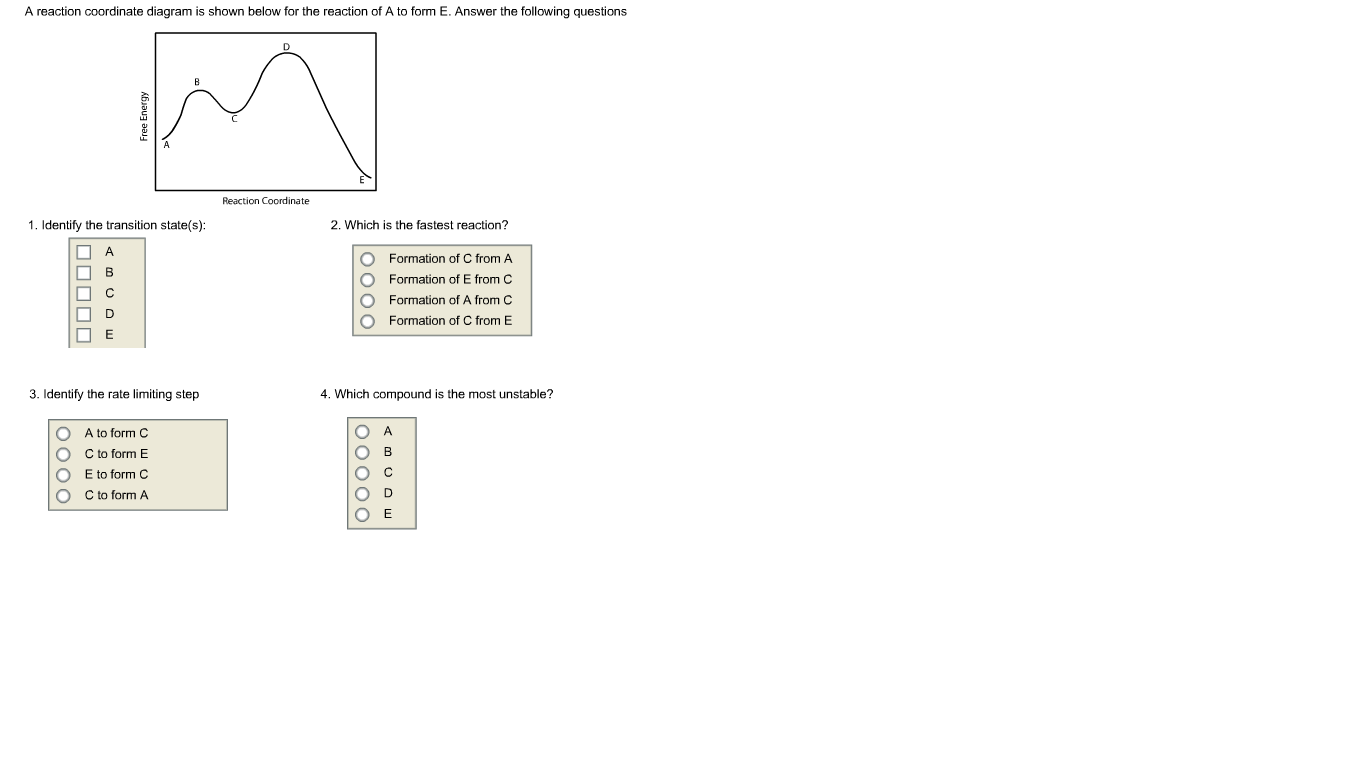
Transcribed Image Text:A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions
Reaction Coordinate
1. Identify the transition state(s):
2. Which is the fastest reaction?
Formation of C from A
В
Formation of E from C
Formation of A from C
D
O Formation of C from E
3. Identify the rate limiting step
4. Which compound is the most unstable?
A to form C
OC to form E
E to form C
C to form A
OooOo
OO000
Free Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1
VIEWTrending now
This is a popular solution!
Step by step
Solved in 1 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning