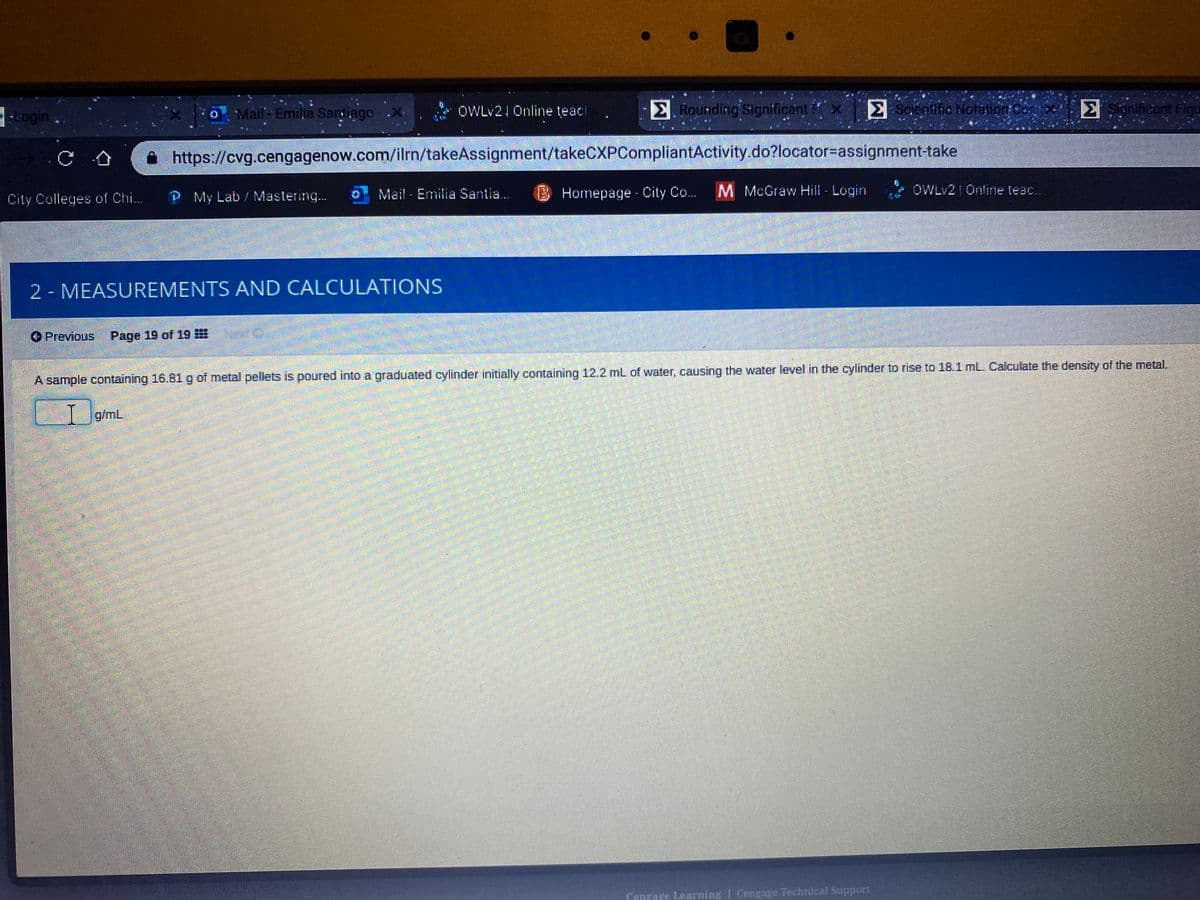
A sample containing 16.81 g of metal pellets is poured into a graduated cylinder initially containing 12.2 mL of water, causing the water level in the cylinder to rise to 18.1 mL. Calculate the density of the metal. g/mL
A sample containing 16.81 g of metal pellets is poured into a graduated cylinder initially containing 12.2 mL of water, causing the water level in the cylinder to rise to 18.1 mL. Calculate the density of the metal. g/mL
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 12P
Related questions
Question
Transcribed Image Text:Mail-Emilia Santiago
OWLV21 Online teac
E. Rounding Significant X E Sejentiio Notatig
Sonificant Figu
ogin.
https://cvg.cengagenow.com/ilrn/takeAssignment/takeCXPCompliantActivity.do?locator%3Dassignment-take
P My Lab/ Mastering...
Mail Emilia Santia..
BHomepage City Co...
M McGraw Hill Login
* OWLV2 | Online teac...
City Colleges of Chi..
2 - MEASUREMENTS AND CALCULATIONS
O Previous Page 19 of 19 H
A sample containing 16.81g of metal pellets is poured into a graduated cylinder initially containing 12.2 mL of water, causing the water level in the cylinder to rise to 18.1 mL. Calculate the density of the metal.
I g/mL
Cengage Learning Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
