A sample of an unknown compound is vaporized at 150. °C. The gas produced has a volume of 2000. mL at a pressure of 1.00 atm, and it weighs 6.00 g. Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Be sure your answer has the correct number of significant digits. g mol x10 X ? Submit Assignment PY Tof Uhe Continue MacBook Pro FA12 FR F7 F6 F5 * F4 F3 F2 F1 esc 5 4 2
A sample of an unknown compound is vaporized at 150. °C. The gas produced has a volume of 2000. mL at a pressure of 1.00 atm, and it weighs 6.00 g. Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Be sure your answer has the correct number of significant digits. g mol x10 X ? Submit Assignment PY Tof Uhe Continue MacBook Pro FA12 FR F7 F6 F5 * F4 F3 F2 F1 esc 5 4 2
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.43QP: A McLeod gauge measures low gas pressures by compressing a known volume of the gas at constant...
Related questions
Question
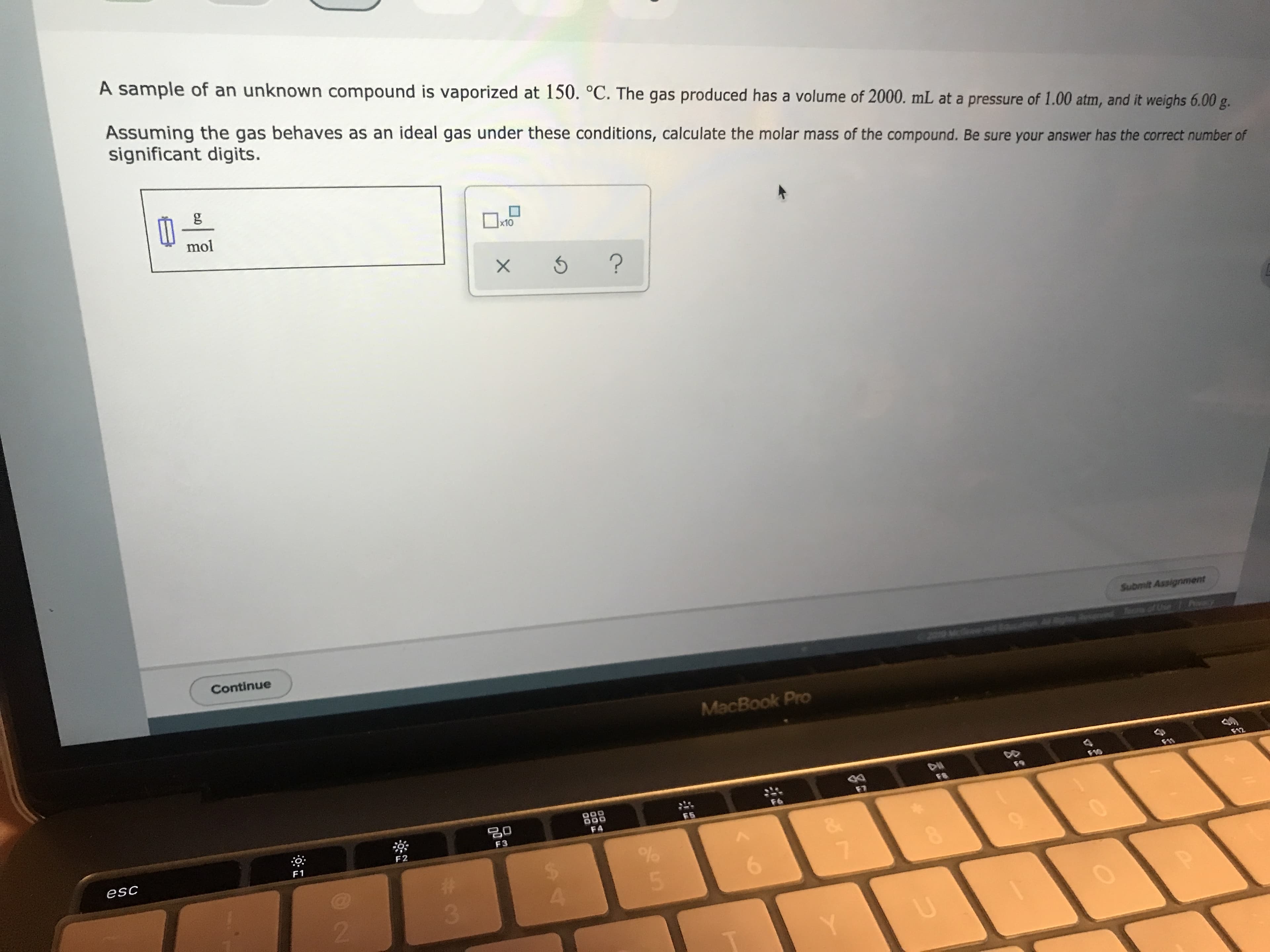
Transcribed Image Text:A sample of an unknown compound is vaporized at 150. °C. The gas produced has a volume of 2000. mL at a pressure of 1.00 atm, and it weighs 6.00 g.
Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Be sure your answer has the correct number of
significant digits.
g
mol
x10
X
?
Submit Assignment
PY
Tof Uhe
Continue
MacBook Pro
FA12
FR
F7
F6
F5
*
F4
F3
F2
F1
esc
5
4
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co