A set of three nucleophilic displacement reactions is shown below: CH3 SOH CH3CH2CCH3 SN1 reaction Br А, SOH %3D 100% CH,ОН В, SOH 3 33% Cн,он, 67% H20 С, SOH %3D 67% CH3ОН, 33% H20 Which reaction (A, B, or C) proceeds the fastest? Which reaction (A, B, or C) proceeds the slowest?
A set of three nucleophilic displacement reactions is shown below: CH3 SOH CH3CH2CCH3 SN1 reaction Br А, SOH %3D 100% CH,ОН В, SOH 3 33% Cн,он, 67% H20 С, SOH %3D 67% CH3ОН, 33% H20 Which reaction (A, B, or C) proceeds the fastest? Which reaction (A, B, or C) proceeds the slowest?
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
100%
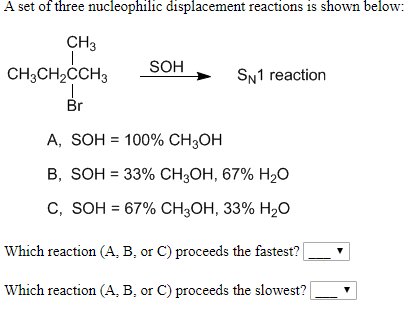
Transcribed Image Text:A set of three nucleophilic displacement reactions is shown below:
CH3
SOH
CH3CH2CCH3
SN1 reaction
Br
А, SOH %3D 100% CH,ОН
В, SOH 3 33% Cн,он, 67% H20
С, SOH %3D 67% CH3ОН, 33% H20
Which reaction (A, B, or C) proceeds the fastest?
Which reaction (A, B, or C) proceeds the slowest?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning