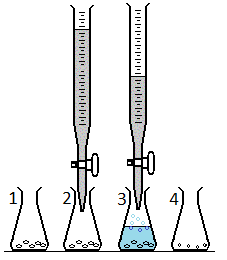
a small amount of marble chips (CaCO3) are placed inside a flask and weighed (as seen in #1 above). Nitric acid (HNO3) is added with a buret causing a reaction to occur (as seen in 2&3 above). After the reaction ends, the liquid is drained away, the remaining chips rinsed and dried (seen in #4 above). The data collected is as follows: * Mass of flask with chips before reaction: 120.15 g
a small amount of marble chips (CaCO3) are placed inside a flask and weighed (as seen in #1 above).
Nitric acid (HNO3) is added with a buret causing a reaction to occur (as seen in 2&3 above).
After the reaction ends, the liquid is drained away, the remaining chips rinsed and dried (seen in #4 above).
The data collected is as follows:
* Mass of flask with chips before reaction: 120.15 g
* Mass of flask with chips after reaction: 112.88 g
* Buret reading before acid is added: 47.07 mL
* Buret reading after acid is added: 22.70 mL
Use the above data to determine the molarity of the nitric acid (HNO3) if the equation for the reaction is:
2HNO3(aq) + CaCO3(s) → H2O(l) + CO2(g) + Ca(NO3)2(aq)
(round your final answer to 2 decimal places)
Step by step
Solved in 2 steps


