A) Standard solution and Beer's law calibration curve 1-Fill in the following table : Concentration For S.1 M¸ x V, M, x V 0.000725 x 1.0ml M, x 10.0ml Solution Absorbance Concentration S. 1 0.0000 725 S.2 0.524 0.789 0,989 0.259 0.000 253 0.000 487 0.0000725 ??? 0.0000552 S 3 S.4 ASA of unknown 2- Plot a graph of Absorbance vs. conc mol/L absorbance Absorbance vs. Concentration 16 14 1.2 0.8 0.6 OY-Values 0.4 0.2 2. 10 conc 10 mol/L B) Analysis of unknown aspirin tablet 1. Mass of aspirin tablet-
A) Standard solution and Beer's law calibration curve 1-Fill in the following table : Concentration For S.1 M¸ x V, M, x V 0.000725 x 1.0ml M, x 10.0ml Solution Absorbance Concentration S. 1 0.0000 725 S.2 0.524 0.789 0,989 0.259 0.000 253 0.000 487 0.0000725 ??? 0.0000552 S 3 S.4 ASA of unknown 2- Plot a graph of Absorbance vs. conc mol/L absorbance Absorbance vs. Concentration 16 14 1.2 0.8 0.6 OY-Values 0.4 0.2 2. 10 conc 10 mol/L B) Analysis of unknown aspirin tablet 1. Mass of aspirin tablet-
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
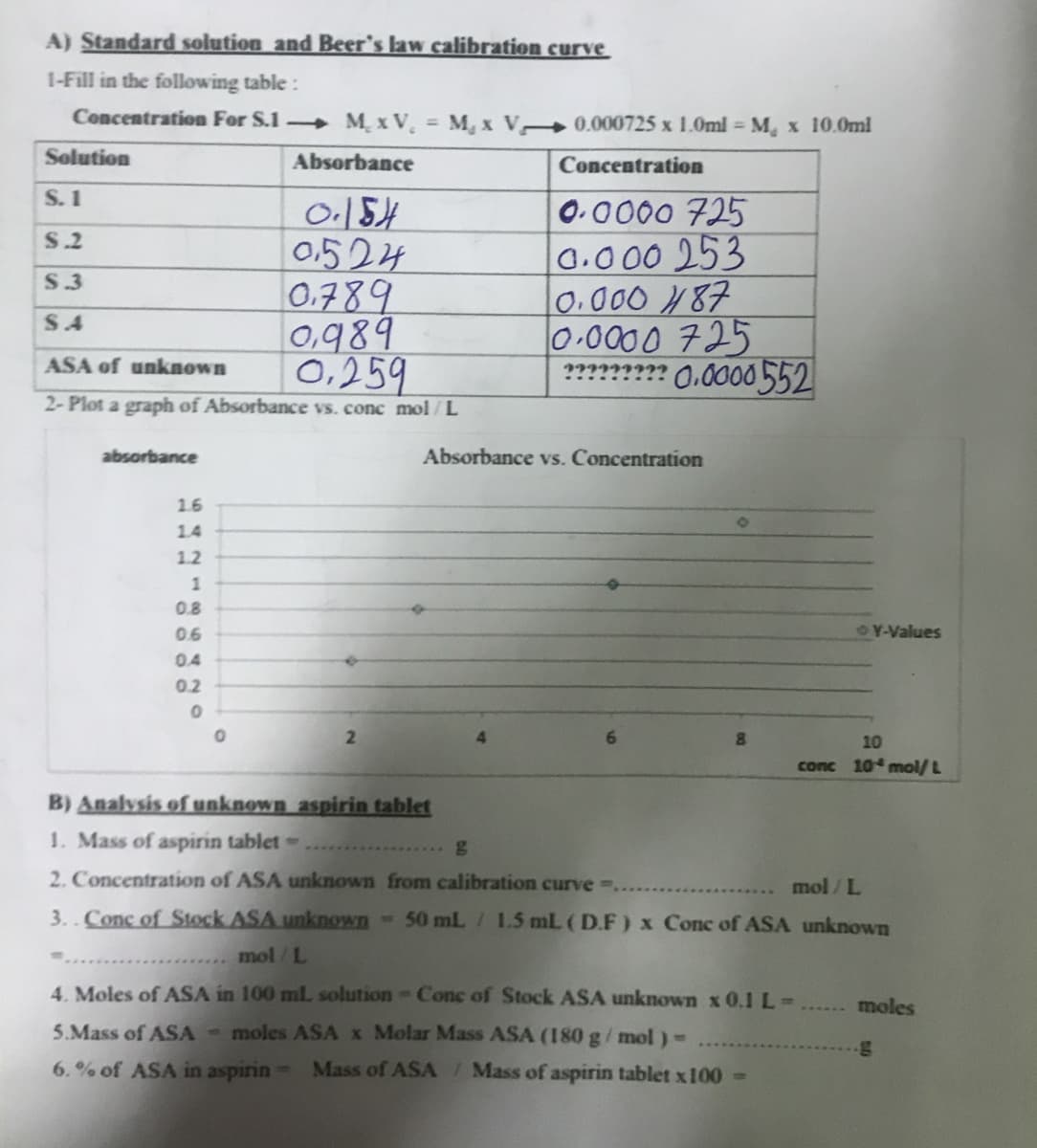
Transcribed Image Text:A) Standard solution and Beer's law calibration curve
1-Fill in the following table :
Concentration For S.1 M. x V. =
M, x V 0.000725 x 1.0ml = M, x 10.0ml
Solution
Absorbance
Concentration
S. 1
0.154
0.524
0.789
0,989
0.259
0.0000 725
0.000 253
0.000 487
0-0000 725
???? 0.0000552
S.2
S.3
S4
ASA of unknown
2- Plot a graph of Absorbance vs. conc mol/L
absorbance
Absorbance vs. Concentration
1.6
14
1.2
1
0.8
0.6
OY-Values
0.4
0.2
2.
10
conc 10 mol/ L
B) Analysis of unknown aspirin tablet
1. Mass of aspirin tablet-
2. Concentration of ASA unknown from calibration curve ..
mol / L
3.. Conc of Stock ASA unknown
-50 mL/ 1.5 mL (D.F) x Conc of ASA unknown
mol/L
4. Moles of ASA in 100 mlL solution Conc of Stock ASA unknown x 0.1 L-
moles
5.Mass of ASA - moles ASA x Molar Mass ASA (180 g/ mol)-
6.% of ASA in aspirin
Mass of ASA/ Mass of aspirin tablet x100 >
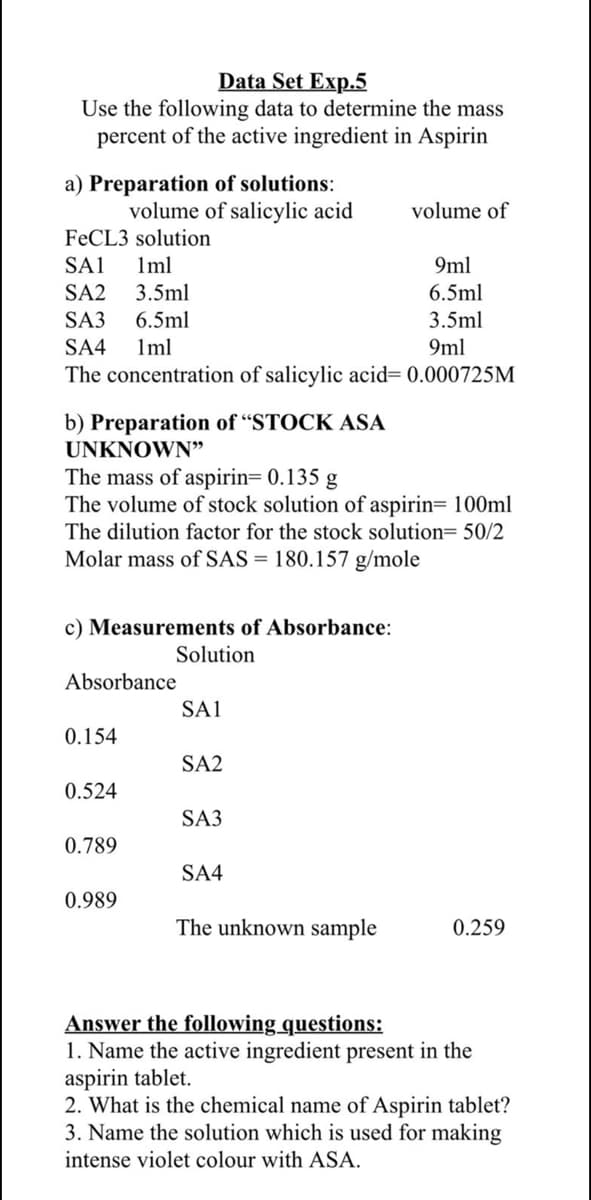
Transcribed Image Text:Data Set Exp.5
Use the following data to determine the mass
percent of the active ingredient in Aspirin
a) Preparation of solutions:
volume of salicylic acid
volume of
FECL3 solution
1ml
3.5ml
SA1
9ml
SA2
6.5ml
SA3
6.5ml
3.5ml
SA4
1ml
9ml
The concentration of salicylic acid= 0.000725M
b) Preparation of “STOCK ASA
UNKNOWN"
The mass of aspirin= 0.135
The volume of stock solution of aspirin= 100ml
g
The dilution factor for the stock solution= 50/2
Molar mass of SAS = 180.157 g/mole
c) Measurements of Absorbance:
Solution
Absorbance
SA1
0.154
SA2
0.524
SA3
0.789
SA4
0.989
The unknown sample
0.259
Answer the following questions:
1. Name the active ingredient present in the
aspirin tablet.
2. What is the chemical name of Aspirin tablet?
3. Name the solution which is used for making
intense violet colour with ASA.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

