A substrate is decomposed in the presence of an enzyme according to the Michaelis-Menten equation with the following kinetic parameters Km-24 g/L Vmax 12 g/L-min a. Determine the concentration of substrate leaving the second reactor in a two-reactor series of 20-liter CSTRs. The flow rate is 3.60 L/min. The inlet substrate concentration is 72 g The enzyme concentration in the two reactors is maintained at the same value all of the time. b. Determine the efficiency in terms of substrate conversion of the two-reactor system and the efficiency of a single CSTR equal to the total volume of the two-reactor system. Clearly indicate the configuration with the greater efficiency.i
A substrate is decomposed in the presence of an enzyme according to the Michaelis-Menten equation with the following kinetic parameters Km-24 g/L Vmax 12 g/L-min a. Determine the concentration of substrate leaving the second reactor in a two-reactor series of 20-liter CSTRs. The flow rate is 3.60 L/min. The inlet substrate concentration is 72 g The enzyme concentration in the two reactors is maintained at the same value all of the time. b. Determine the efficiency in terms of substrate conversion of the two-reactor system and the efficiency of a single CSTR equal to the total volume of the two-reactor system. Clearly indicate the configuration with the greater efficiency.i
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
PLease show all steps
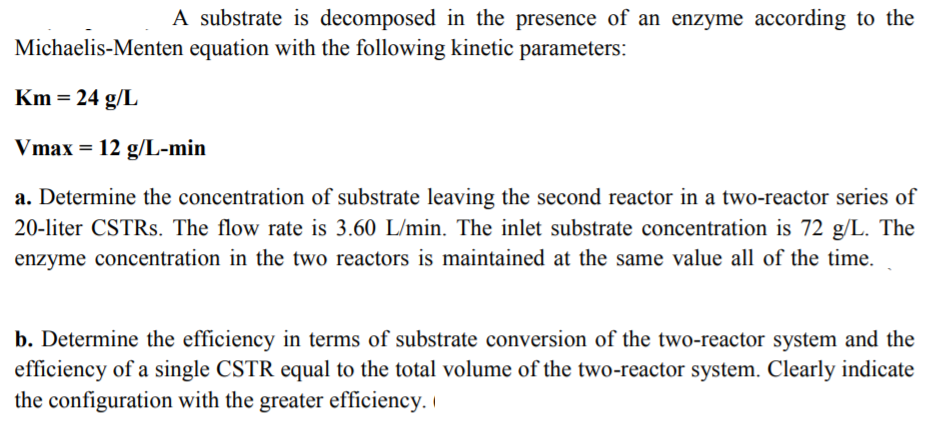
Transcribed Image Text:A substrate is decomposed in the presence of an enzyme according to the
Michaelis-Menten equation with the following kinetic parameters
Km-24 g/L
Vmax 12 g/L-min
a. Determine the concentration of substrate leaving the second reactor in a two-reactor series of
20-liter CSTRs. The flow rate is 3.60 L/min. The inlet substrate concentration is 72 g The
enzyme concentration in the two reactors is maintained at the same value all of the time.
b. Determine the efficiency in terms of substrate conversion of the two-reactor system and the
efficiency of a single CSTR equal to the total volume of the two-reactor system. Clearly indicate
the configuration with the greater efficiency.i
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The