A voltaic cell is constructed from a standard H*|H, half cell (E°, red = 0.000V) and a standard Cl,|CIr half cell (E°, red = 1.360V). The anode reaction is: +I+ +|+ The cathode reaction is: The spontaneous cell reaction is: The cell voltage is V.
A voltaic cell is constructed from a standard H*|H, half cell (E°, red = 0.000V) and a standard Cl,|CIr half cell (E°, red = 1.360V). The anode reaction is: +I+ +|+ The cathode reaction is: The spontaneous cell reaction is: The cell voltage is V.
Chapter19: Applications Of Standard Electrode Potentials
Section: Chapter Questions
Problem 19.8QAP
Related questions
Question
100%
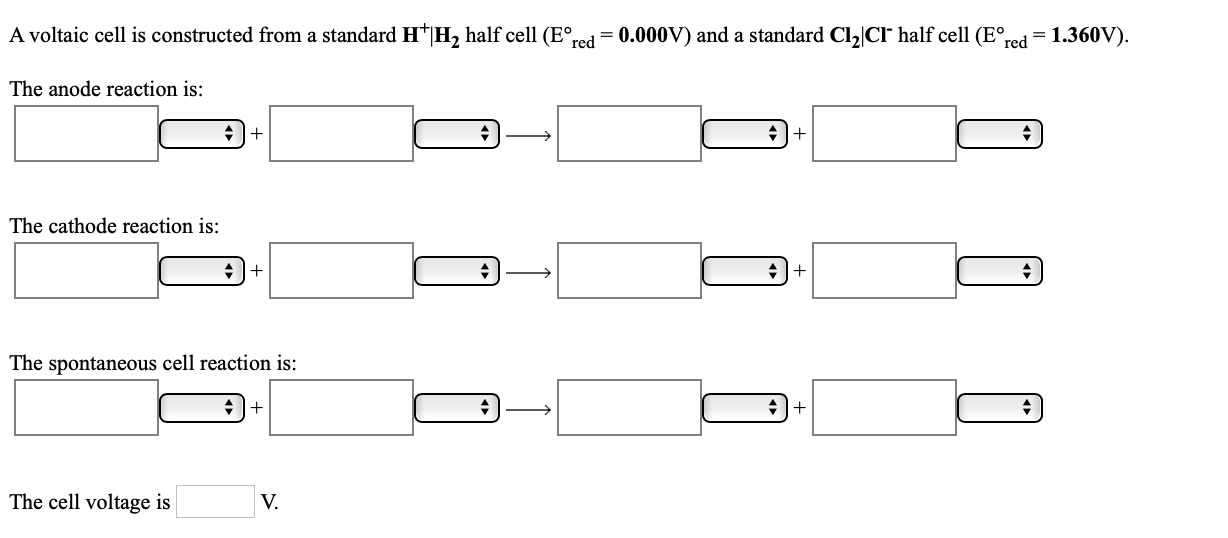
Transcribed Image Text:A voltaic cell is constructed from a standard H*|H, half cell (E°,
red
= 0.000V) and a standard Cl,|CIr half cell (E°,
red
= 1.360V).
The anode reaction is:
+I+
+|+
The cathode reaction is:
The spontaneous cell reaction is:
The cell voltage is
V.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning