a. Naphthalene is a white volatile, solid polycyclic hydrocarbon used in moth balls and repellents. Looking at the structure of naphthalene given below, determine if it is aromatic or not. Explain the reasons for your answer? b. The table below shows the boiling point points of an alkane and aldehyde and an alcohol:
a. Naphthalene is a white volatile, solid polycyclic hydrocarbon used in moth balls and repellents. Looking at the structure of naphthalene given below, determine if it is aromatic or not. Explain the reasons for your answer? b. The table below shows the boiling point points of an alkane and aldehyde and an alcohol:
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter12: Unsaturated Hydrocarbons
Section: Chapter Questions
Problem 12.72E
Related questions
Question
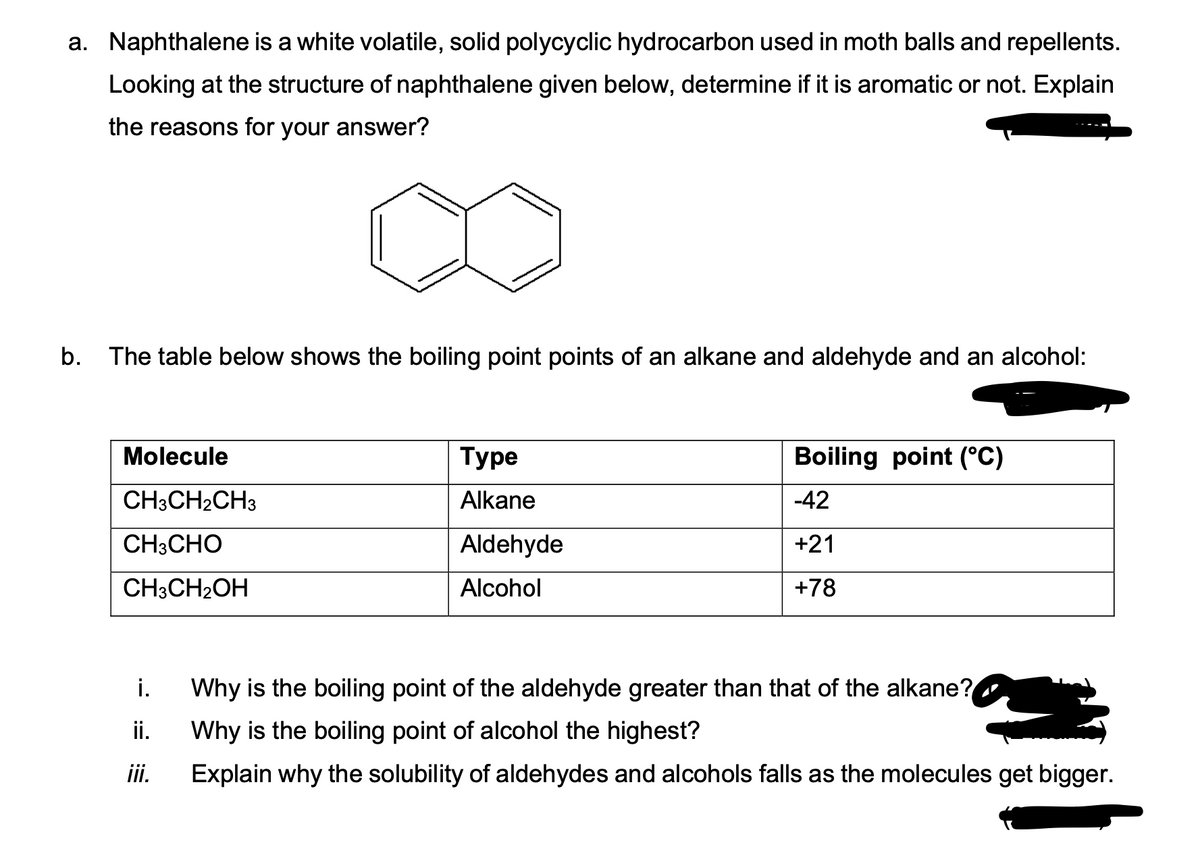
Transcribed Image Text:a. Naphthalene is a white volatile, solid polycyclic hydrocarbon used in moth balls and repellents.
Looking at the structure of naphthalene given below, determine if it is aromatic or not. Explain
the reasons for your answer?
b. The table below shows the boiling point points of an alkane and aldehyde and an alcohol:
Molecule
CH3CH₂CH3
CH3CHO
CH3CH₂OH
i.
ii.
iii.
Туре
Alkane
Aldehyde
Alcohol
Boiling point (°C)
-42
+21
+78
Why is the boiling point of the aldehyde greater than that of the alkane?
Why is the boiling point of alcohol the highest?
Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning