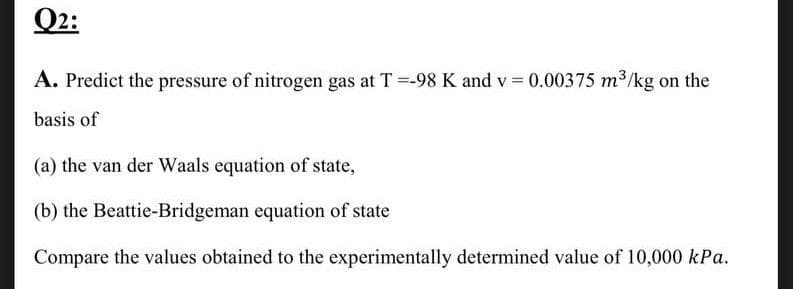
A. Predict the pressure of nitrogen gas at T =-98 K and v=0.00375 m³/kg on the basis of (a) the van der Waals equation of state, (b) the Beattie-Bridgeman equation of state Compare the values obtained to the experimentally determined value of 10,000 kPa.
A. Predict the pressure of nitrogen gas at T =-98 K and v=0.00375 m³/kg on the basis of (a) the van der Waals equation of state, (b) the Beattie-Bridgeman equation of state Compare the values obtained to the experimentally determined value of 10,000 kPa.
Biomedical Instrumentation Systems
1st Edition
ISBN:9781133478294
Author:Chatterjee
Publisher:Chatterjee
Chapter11: Instrumentation In Respiration
Section: Chapter Questions
Problem 9Q
Related questions
Question
Transcribed Image Text:Q2:
A. Predict the pressure of nitrogen gas at T =-98 K and v=0.00375 m³/kg on the
basis of
(a) the van der Waals equation of state,
(b) the Beattie-Bridgeman equation of state
Compare the values obtained to the experimentally determined value of 10,000 kPa.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you



