Absorbance Concentration slope (m) 2528.2 Intercept (c) 0.0253 0.0002 0.495655377 0.00015 0.45258486 0.0001 Absorbance of unknown sample 0.262383057 0.040399 Concentration of unknown sample 0.00005 0.179808907 5.97223E-06 0.6 y = 2528.2x+ 0.0253 R? = 0.9683 0.5 0.4 0.3 0.2 0.1 0.00005 0.0001 0.00015 0.0002 0.00025 Concentration (M) Absorbance
Absorbance Concentration slope (m) 2528.2 Intercept (c) 0.0253 0.0002 0.495655377 0.00015 0.45258486 0.0001 Absorbance of unknown sample 0.262383057 0.040399 Concentration of unknown sample 0.00005 0.179808907 5.97223E-06 0.6 y = 2528.2x+ 0.0253 R? = 0.9683 0.5 0.4 0.3 0.2 0.1 0.00005 0.0001 0.00015 0.0002 0.00025 Concentration (M) Absorbance
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.2QAP
Related questions
Question
100%
For my lab I had to find the unknown concentration of a solution. I found wavelength max of the potassium chromate. Then used that max to find the absorbtion for my sample dilutions. Based on my attached graph. Is my unknown concentration # making sense? Or did I make an error with the absorbance I wrote down in the lab. I did my calculations when I got home. They are correct, but my unknown concentration amount doesn't look like it will even be on the graph. Or am I wrong. Please advise and explain. Thank you
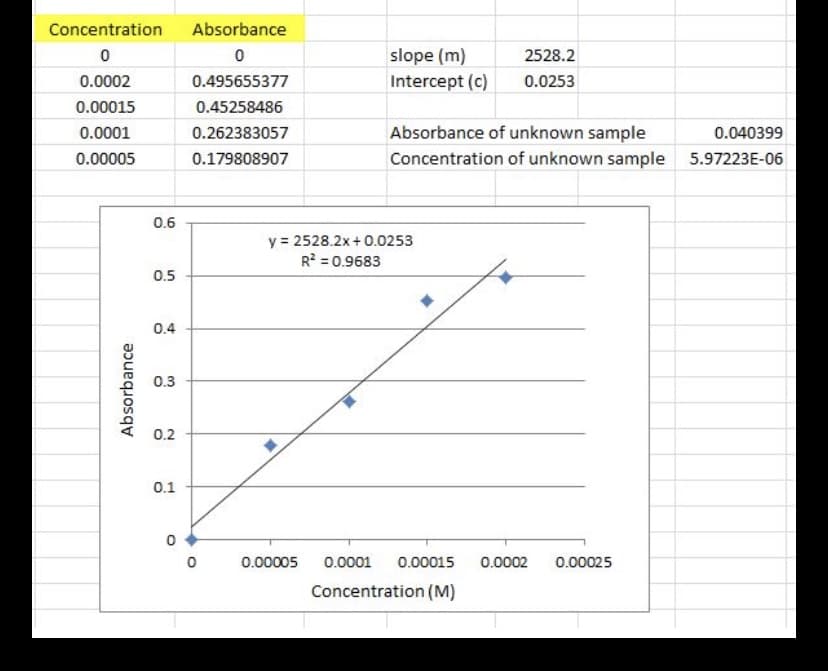
Transcribed Image Text:Absorbance
Concentration
slope (m)
2528.2
Intercept (c)
0.0253
0.0002
0.495655377
0.00015
0.45258486
0.0001
Absorbance of unknown sample
0.262383057
0.040399
Concentration of unknown sample
0.00005
0.179808907
5.97223E-06
0.6
y = 2528.2x+ 0.0253
R? = 0.9683
0.5
0.4
0.3
0.2
0.1
0.00005
0.0001
0.00015
0.0002
0.00025
Concentration (M)
Absorbance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning