According to the following chemical equation, how many liters at STP of hydrogen gas are needed to make 3.00 tons of iron? (2000 lbs. = 1 ton; 454 g = 1 lb.) [Hint: The equation is not balanced.] %D Fe,O3 + Н2 Fe + Н.О omic
According to the following chemical equation, how many liters at STP of hydrogen gas are needed to make 3.00 tons of iron? (2000 lbs. = 1 ton; 454 g = 1 lb.) [Hint: The equation is not balanced.] %D Fe,O3 + Н2 Fe + Н.О omic
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter13: Gases
Section: Chapter Questions
Problem 86A
Related questions
Question
![According to the following chemical equation, how many liters at STP of hydrogen gas are needed
to make 3.00 tons of iron? (2000 lbs. = 1 ton; 454 g = 1 lb.) [Hint: The equation is not balanced.]
%D
Fe,O3 +
Н2
Fe +
Н.О
omic](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fcfef55d1-e74e-4acc-a65e-b6e34bf1c85d%2Fdc01207d-3c72-4f8b-89cf-e0fad70f602b%2F8792yi9.jpeg&w=3840&q=75)
Transcribed Image Text:According to the following chemical equation, how many liters at STP of hydrogen gas are needed
to make 3.00 tons of iron? (2000 lbs. = 1 ton; 454 g = 1 lb.) [Hint: The equation is not balanced.]
%D
Fe,O3 +
Н2
Fe +
Н.О
omic
Expert Solution
Step 1
Amount of iron to be manufactured = 3 tonnes
The given equation for the reaction is

Step 2
mass of iron given = 3ton x 2000lb/ton x 454g/lb= 2724000g
No of moles of iron to be manufactured
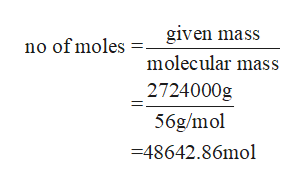
Step 3
Amount of hydrogen needed
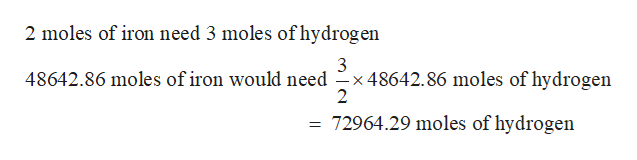
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning