Activation energy for the reaction is 44KJ/mole. A catalyst is added and the reaction rate constant increases from 34L3mole-3s-1 to 400 L3mole-3s-1 . What is then the activation energy given T = 25 degrees celsius?
Activation energy for the reaction is 44KJ/mole. A catalyst is added and the reaction rate constant increases from 34L3mole-3s-1 to 400 L3mole-3s-1 . What is then the activation energy given T = 25 degrees celsius?
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter10: Acids, Bases, And Salts
Section: Chapter Questions
Problem 10.26EP: The formula for tartaric acid is preferably written as H2C4H4O6 rather than as C4H6O6. Explain why.
Related questions
Question
Activation energy for the reaction is 44KJ/mole. A catalyst is added and the reaction rate constant increases from 34L3mole-3s-1 to 400 L3mole-3s-1 . What is then the activation energy given T = 25 degrees celsius?
See picture for reaction
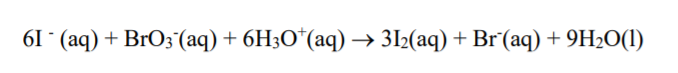
Transcribed Image Text:61 (aq) + BrO3(aq) + 6H3O*(aq)→ 312(aq) + Br´(aq) + 9H2O(1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax