Activities Chromium Web Browser - Jan 23 9:10 AM b My Questions | bartleby O Mail - Dishita Uppal - Out WA HW: IMF, VP, phases, unit x O (1) TextNow W Answers That Cannot Be x A webassign.net/web/Student/Assignment-Responses/last?dep=22994801#Q9 N My Notes + Ask Your Teacher -/3 points 12. A representation of a unit cell for a compound of formula Au Cu, is shown below. (a) Identify the type of unit cell shown. O body-centered cubic O face-centered cubic O simple cubic (b) Determine the number of Au atoms (blue spheres) and Cu atoms (yellow spheres) in a unit cell. Au atoms Cu atoms (C) Determine the empirical formula for Au Cu (Omit states-of-matter from your answer.) O Help chemPad Greek 123 Tutorial
Activities Chromium Web Browser - Jan 23 9:10 AM b My Questions | bartleby O Mail - Dishita Uppal - Out WA HW: IMF, VP, phases, unit x O (1) TextNow W Answers That Cannot Be x A webassign.net/web/Student/Assignment-Responses/last?dep=22994801#Q9 N My Notes + Ask Your Teacher -/3 points 12. A representation of a unit cell for a compound of formula Au Cu, is shown below. (a) Identify the type of unit cell shown. O body-centered cubic O face-centered cubic O simple cubic (b) Determine the number of Au atoms (blue spheres) and Cu atoms (yellow spheres) in a unit cell. Au atoms Cu atoms (C) Determine the empirical formula for Au Cu (Omit states-of-matter from your answer.) O Help chemPad Greek 123 Tutorial
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.25E: 6.25. Phosphorus exists as several allotropes that have varying properties. The enthalpy of...
Related questions
Question
Transcribed Image Text:Activities
Chromium Web Browser -
Jan 23 9:10 AM
b My Questions | bartleby
O Mail - Dishita Uppal - Out
WA HW: IMF, VP, phases, unit x
O (1) TextNow
W Answers That Cannot Be
x
A webassign.net/web/Student/Assignment-Responses/last?dep=22994801#Q9
N My Notes
+ Ask Your Teacher
-/3 points
12.
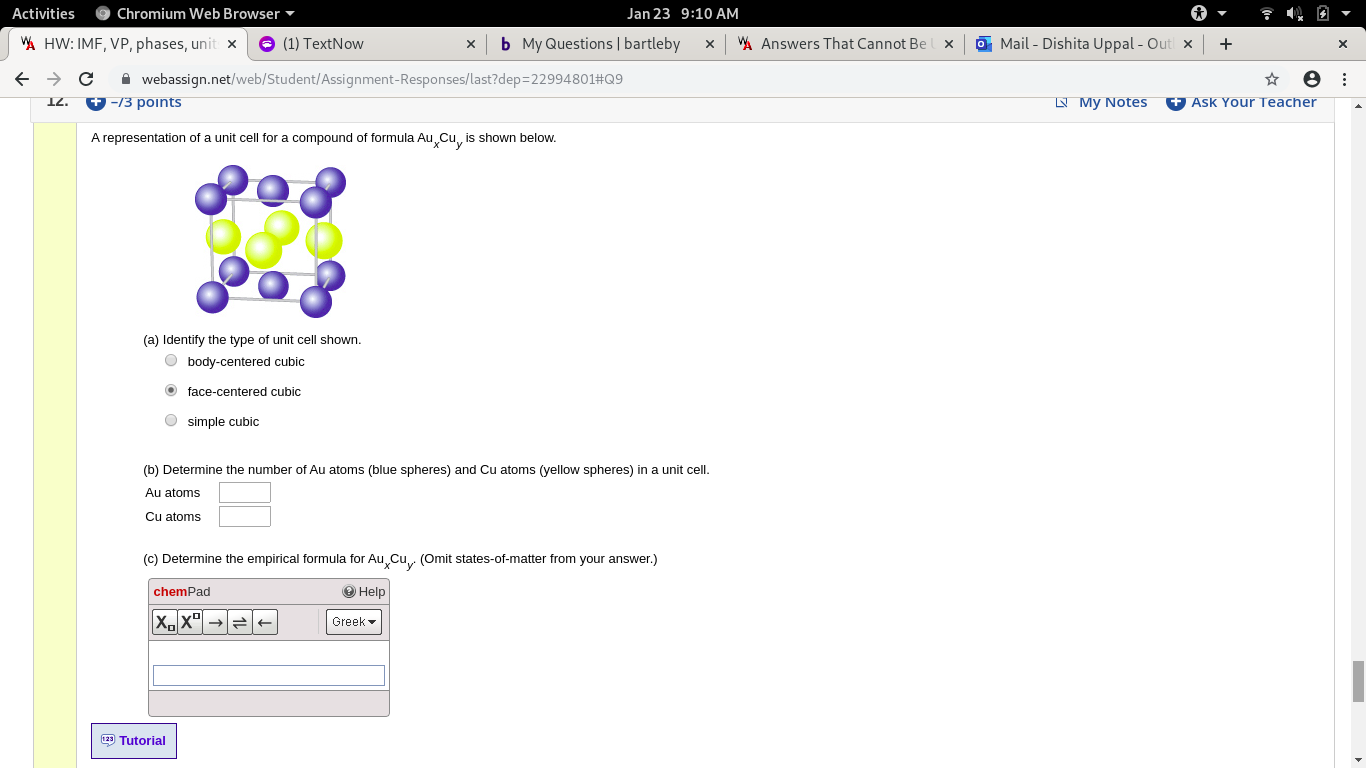
A representation of a unit cell for a compound of formula Au Cu, is shown below.
(a) Identify the type of unit cell shown.
O body-centered cubic
O face-centered cubic
O simple cubic
(b) Determine the number of Au atoms (blue spheres) and Cu atoms (yellow spheres) in a unit cell.
Au atoms
Cu atoms
(C) Determine the empirical formula for Au Cu
(Omit states-of-matter from your answer.)
O Help
chemPad
Greek
123 Tutorial
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax