ACTIVITY 1: MASS RELATIONSHIPS IN CHEMICAL REACTIONS Name: Section: DIRECTIONS: Answer the following problems. Write your complete solution on a separate sheet of paper. 1. The combustion of carbon monoxide gas in oxygen gas is represented by the following balanced equation: 2 CO(g) + O2(g) 2CO2(g) How many moles of carbon dioxide gas will be produced from the complete combustion of 4.60 moles CO(g)? 2. Consider the reaction: 2 KCIO3 → 2 KCI + 3 O2 a. How many moles of KCIO3 are required to produce 22.8 moles oxygen gas, O2? b. How many moles of KCI will be produced from the total decomposition of 18.8 moles KCIO3? 3. Silver metal reacts with sulfur sulfide according to the following reaction: 2Ag (s) + S(s) → Ag2S(s) a. Identify the reagent if 50.0 g Ag reacts with 10.0 g S. b. What is the theoretical yield in g of Ag2S produced from the reaction? c. What is the amount in g of the excess reactant expected to remain after the reaction? d. When the reaction occurred, the amount of Ag2S obtained was 45.0 g. What is the percent yield of the reaction?
ACTIVITY 1: MASS RELATIONSHIPS IN CHEMICAL REACTIONS Name: Section: DIRECTIONS: Answer the following problems. Write your complete solution on a separate sheet of paper. 1. The combustion of carbon monoxide gas in oxygen gas is represented by the following balanced equation: 2 CO(g) + O2(g) 2CO2(g) How many moles of carbon dioxide gas will be produced from the complete combustion of 4.60 moles CO(g)? 2. Consider the reaction: 2 KCIO3 → 2 KCI + 3 O2 a. How many moles of KCIO3 are required to produce 22.8 moles oxygen gas, O2? b. How many moles of KCI will be produced from the total decomposition of 18.8 moles KCIO3? 3. Silver metal reacts with sulfur sulfide according to the following reaction: 2Ag (s) + S(s) → Ag2S(s) a. Identify the reagent if 50.0 g Ag reacts with 10.0 g S. b. What is the theoretical yield in g of Ag2S produced from the reaction? c. What is the amount in g of the excess reactant expected to remain after the reaction? d. When the reaction occurred, the amount of Ag2S obtained was 45.0 g. What is the percent yield of the reaction?
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
Section: Chapter Questions
Problem 12RE
Related questions
Question
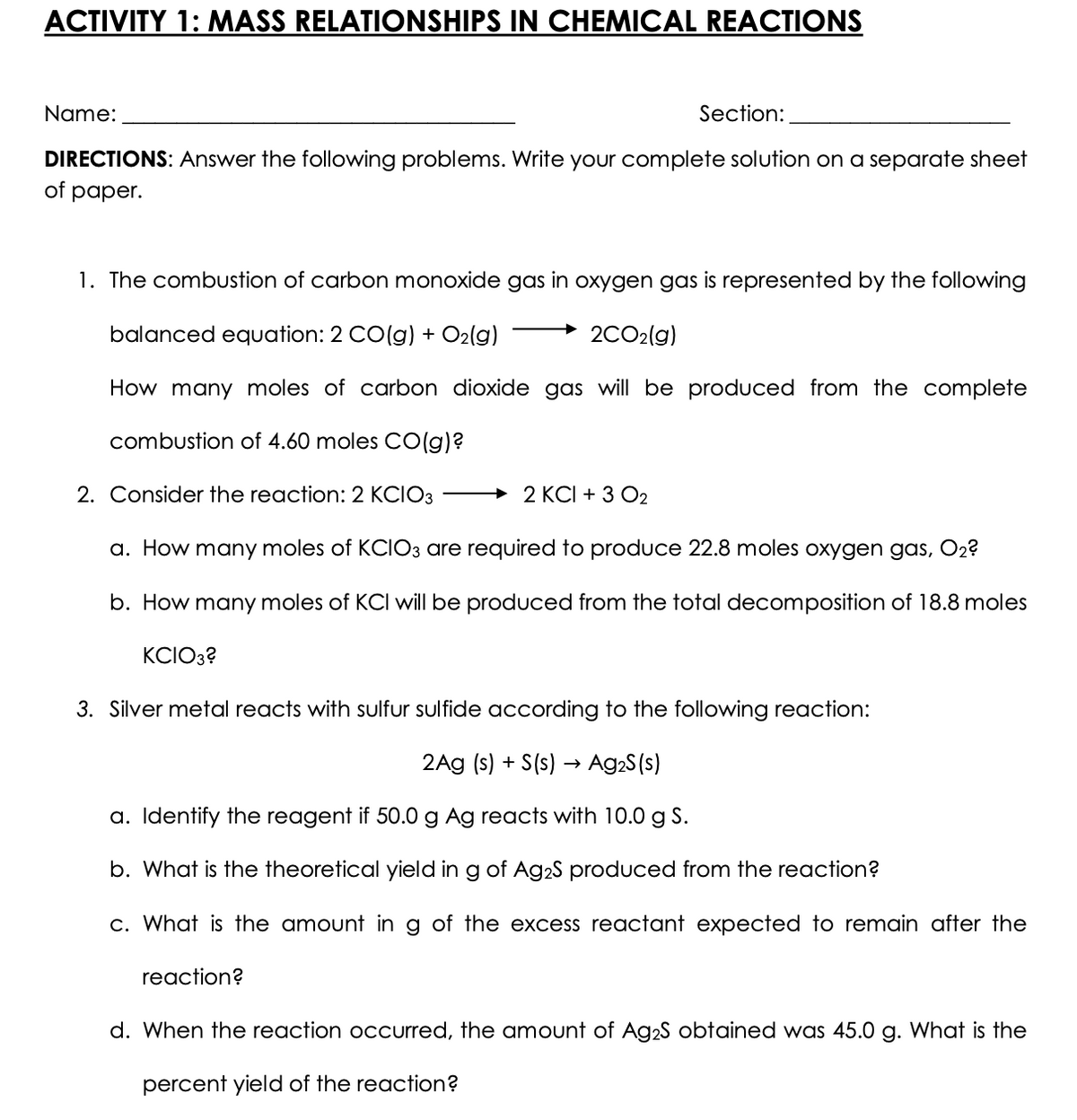
Transcribed Image Text:ACTIVITY 1: MASS RELATIONSHIPS IN CHEMICAL REACTIONS
Name:
Section:
DIRECTIONS: Answer the following problems. Write your complete solution on a separate sheet
of paper.
1. The combustion of carbon monoxide gas in oxygen gas is represented by the following
balanced equation: 2 CO(g) + O2(g)
2CO2(g)
How many moles of carbon dioxide gas will be produced from the complete
combustion of 4.60 moles CO(g)?
2. Consider the reaction: 2 KCIO3
+ 2 KCI + 3 O2
a. How many moles of KCIO3 are required to produce 22.8 moles oxygen gas, O2?
b. How many moles of KCI will be produced from the total decomposition of 18.8 moles
KCIO3?
3. Silver metal reacts with sulfur sulfide according to the following reaction:
2Ag (s) + S(s) → Ag2S (s)
a. Identify the reagent if 50.0g Ag reacts with 10.0 g S.
b. What is the theoretical yield in g of Ag2S produced from the reaction?
c. What is the amount in g of the excess reactant expected to remain after the
reaction?
d. When the reaction occurred, the amount of Ag2S obtained was 45.0 g. What is the
percent yield of the reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
