Actual wavelength of the bright green line from Observed wavelength of the bright green line REPORT 85 ATOMIC EMISSION SPECTROSCOPY Jamin GoaLeleer NAME Analysis of the Hydrogen Spectrum. SECTION the fluorescent lights, (nm) 546 from the fluorescent lights, (am) Calibration factor, (nm) Hydrogen Observed Wavelength (nm) Corrected wavelength (nm) GE4 Corrected wavelength (m) Initial n value Color 3-72 b.54x 10-7 4.86 Y 10-m4>2 Red Green 488 blue 438 4.3@ x 10 m5-2 hPurple 413 4.13 X10-9 m6->2 Data to be plotted for graphical method Inverse of initial n value (in decimal form) Inverse of wavelength (m) R, (in m-) from the slope of line plotted in the graphical method Percent error, (%) CALCULATIONS (use separate sheets to show your work) b54 x 10 4 1 ESY * 10 9m
Actual wavelength of the bright green line from Observed wavelength of the bright green line REPORT 85 ATOMIC EMISSION SPECTROSCOPY Jamin GoaLeleer NAME Analysis of the Hydrogen Spectrum. SECTION the fluorescent lights, (nm) 546 from the fluorescent lights, (am) Calibration factor, (nm) Hydrogen Observed Wavelength (nm) Corrected wavelength (nm) GE4 Corrected wavelength (m) Initial n value Color 3-72 b.54x 10-7 4.86 Y 10-m4>2 Red Green 488 blue 438 4.3@ x 10 m5-2 hPurple 413 4.13 X10-9 m6->2 Data to be plotted for graphical method Inverse of initial n value (in decimal form) Inverse of wavelength (m) R, (in m-) from the slope of line plotted in the graphical method Percent error, (%) CALCULATIONS (use separate sheets to show your work) b54 x 10 4 1 ESY * 10 9m
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
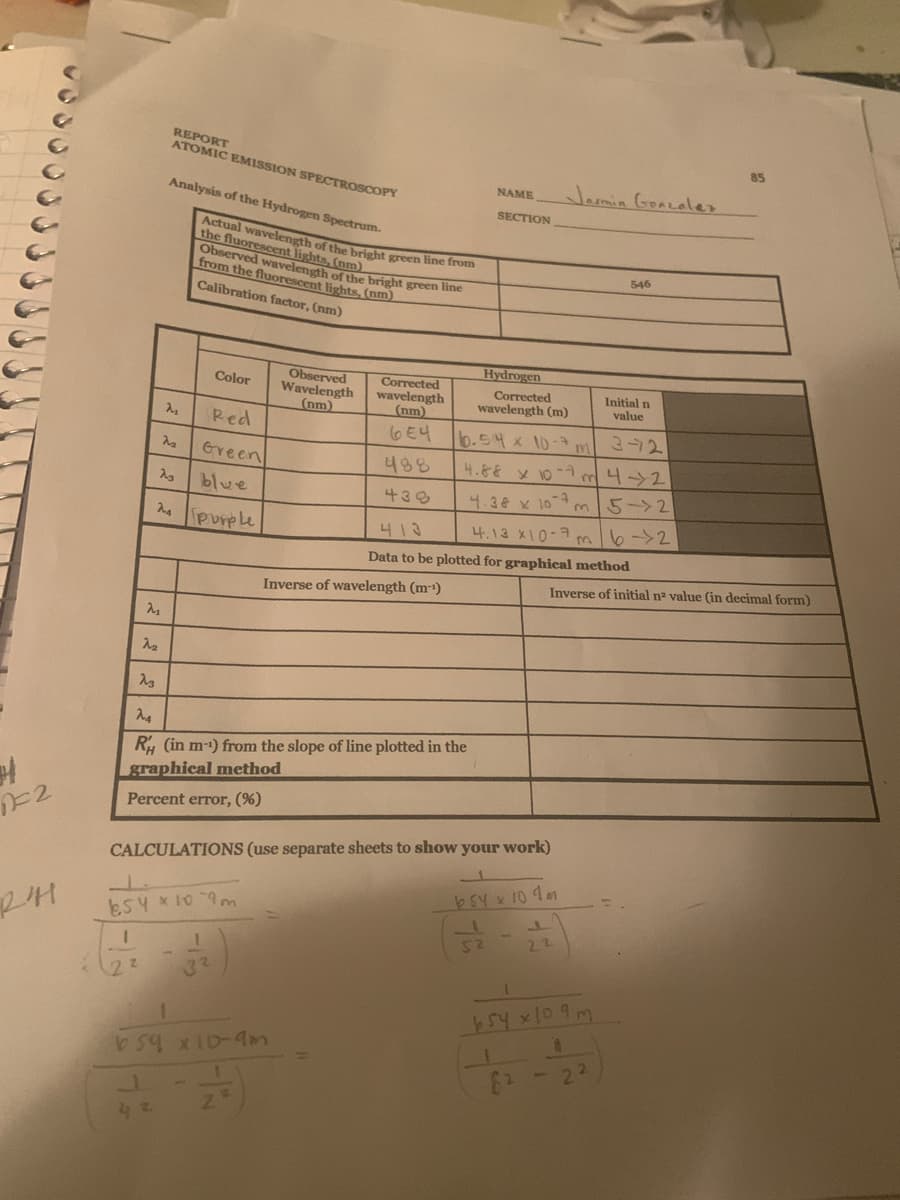
Transcribed Image Text:REPORT
ATOMIC EMISSION SPECTROSCOPY
85
Jamin GoaLeler
NAME
Analysis of the Hydrogen Spectrum.
SECTION
Actual wavelength of the bright green line from
the fluorescent lights, (nm)
Observed wavelength of the bright green ihe
from the fluorescent lights, (nm)
546
Calibration factor, (nm)
Hydrogen
Observed
Wavelength
(nm)
Corrected
wavelength
(nm)
GE4
Color
Corrected
wavelength (m)
Initial n
value
Red
6.54x 10-7m372
4.86 x 10-7 m4>2
Green
488
blue
430
4.38 x 10 m 5->2
Purple
4.13 X10-7 m6->2
413
Data to be plotted for graphical method
Inverse of initial na value (in decimal form)
Inverse of wavelength (m-)
R, (in m-1) from the slope of line plotted in the
graphical method
Percent error, (%)
CALCULATIONS (use separate sheets to show your work)
b54 x 10 4 1
esy *10 9m
52
22
32
b59 x1D-9m
21
1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning