An electron is moving with a velocity of 2.56 x10’ m/s. Calculate the minimum possible uncertainty in its position. (a) 0.038×10-12 m c (b) 4.50 x10-10 (c) 4.50 x10-12 m (d) None of the above m
Q: A stretched string tied at both ends is oscillating in its second harmonic. The string’s shape is de...
A:
Q: Problem 2: Refrigerator Suppose that heat leaks into your kitchen refrigerator at an average rate of...
A:
Q: Problem 1: Estimate the probability that a hydrogen atom at room temperature is in one of its first ...
A: Basic Details The energy difference on the first state of energy can be determined as it depends on ...
Q: A meter stick is moving towards right with a speed 0.9c . The stick is making angle 23° above the po...
A:
Q: Problem 17: Two small loudspeakers, L1 and L2, are oscillating in phase at a frequency of 516 Hz, se...
A:
Q: 4 An infinitely long cylinder of radius R has magnetization M = Mo a) Find the magnetic field B ever...
A: The magnetization of an infinitely long cylinder of radius R is, The volume bound current J is give...
Q: For this problem, I know the answer is B but I do not know how to get there. Seeing the steps and eq...
A:
Q: A large room contains a volume of air of Va = 15 m3 at a temperature of Ta = 53° F. It also contains...
A:
Q: Problem 1: Fermi temperature of the sun At the center of the sun, the temperature is approximately 1...
A:
Q: (b) The density of iron is 7.85 g/cm3, and its atomic mass is 55.8. Assuming each iron atom contribu...
A: Hey, since there is multiple subpart question posted, we will answer first three question. If you wa...
Q: A 0.029-kg bullet is fired vertically at 240 m/s into a 0.15-kg baseball that is initially at rest. ...
A: Let the mass of the bullet be denoted as m and let the mass of the baseball be denoted as M. Let th...
Q: A(n) 878 kg drag race car accelerates from rest to 102 km/h in 0.962 s. What average force is exerte...
A:
Q: please ONLY redo #6
A: Let the mass of the protons be denoted by m each. From the Newton’s universal law of gravitation, th...
Q: if the star had an output power of 1.22x10^27W, a) what is the average intensity at a distance of 2x...
A: Hey, since there are multiple subparts question posted, we will answer first three questions. If you...
Q: Quick Quiz 23.4 object A has a charge of +2 uC, and object B has a charge of +6 µC. Which statement ...
A:
Q: A monoatomic ideal gas is taken through the cycle A→B→C→A shown in the figure. Express all the answe...
A: Basic Details The work done in the process A-B can be determined as the product of pressure at point...
Q: Imagine a football has been stored in a warm locker room at a temperature of 71 F, and has a gauge p...
A:
Q: A spaceship's orbital maneuver requires a speed increase of 1.30 ✕ 103 m/s. If its engine has an exh...
A:
Q: please help has two parts
A:
Q: Please show work neatly, thank you.
A:
Q: How do you calculate density of air at 100 degrees Fahrenheit at 40 psia for mass flow rate equation...
A: Basic Details The mass flow rate equation denotes that the mass flow rate is equal to the product of...
Q: For p.37 at phase diagram Fe-Fe3C : •point the temperature and the carbon content; •the ...
A: Basic Details The Fe-Fe3C diagram show the composition of Fe and Fe3C at various temperatures as the...
Q: For this problem, I know the answer is C but I do not know how to get there. Seeing the steps and eq...
A:
Q: A person is in a closed room (a racquetball court) with V = 435 m3 hitting a ball (m = 37.6 g) aroun...
A:
Q: Problem 1: This problem concerns a collection of N identical harmonic oscillators (perhaps an Einste...
A: Since you have posted a question with multiple sub-parts, we will solve the first three subparts for...
Q: Question 1 Unsaved ume the double-slit experiment described in Problem 6 (with the 3rd dark fringe o...
A:
Q: 64 see photo
A:
Q: please do part d
A:
Q: A 2.40kg block is dropped onto a spring from a height of 5.00 m above the top of the spring. When th...
A:
Q: Special Relativity Question John carries a meter stick and runs through a barn door that is 1 meter ...
A: Basic Details The length contraction is a phenomenon of difference is the length frame due to an obj...
Q: I already know the answer to this practice problem, and it is shown is the image. I do not know how ...
A:
Q: A closed and elevated vertical cylindrical tank with diameter 2.20 mm contains water to a depth of 0...
A:
Q: Assignment-3) Calculate the extent of the depletion region and the peak electric field in an Al/n-Si...
A: The Schottky diode is essentially a one sided p-n junction device .Let xn denote the width of the n...
Q: For this problem, I know the answer is C but I do not know how to get there. Seeing the steps and eq...
A: The value of the refractive index of diamond is,
Q: The forces exerted by the bicep muscle and acting on the elbow when a 12 kg barbell is being curled ...
A: The given problem is basically a torque problem. If τ be defined as the net torque acting at the elb...
Q: A 74-kg fisherman in a 118-kg boat throws a package of mass m = 15 kg horizontally toward the right ...
A: Given information: Here, M, mb, and m are the mass of the fisherman, boat and the package respectiv...
Q: brenda pushes a box horizontally with a force of 200 N (F=200N). The mass of the box is 50 kg. Ass...
A:
Q: Complete the following statement: A collision is elastic if the final kinetic energy is zero. the ob...
A: The momentum is conserved in both elastic and inelastic collision. But in a inelastic collision the ...
Q: 24 in. 2 m 24 in 18 in 1 m 1.5 m Prob. 5-66 Prob 5-69. Determine the tens components of reaction at ...
A:
Q: Astronomers use the distance modulus function M(r) = 5 log r − 5, where M is the distance modulus ...
A: Basic Details The distance modulus function is the function that is used to determine the distance o...
Q: A monoatomic ideal gas is taken through the cycle A → B → C → A shown in the figure. If we know ...
A: Basic Details An adiabatic process is a thermodynamic process that occurs without transfer of the ma...
Q: Problem 1: a) Calculate the most probable speed, average speed, and rms speed for nitrogen N, at roo...
A: Part(a) Basic Details The root mean speed of any gas is root of the men of the speed of the all the...
Q: Determine the x-coordinate of the centroid of the shaded area by integration.
A:
Q: Two separate capacitors, C1 and C2 C1 = 36 micro-Coulomb on 3 micro-Farad C2 = 72 uC on X uF, X = ...
A:
Q: The bent wire shown in the figure lies in a uniform magnetic field. Each straight section is 1.46 m ...
A:
Q: (a) Enter the smaller possible value of R. (b) Enter the larger possible value of R.
A:
Q: Problem 9: Two point charges lie on the x-axis: Q1 +4.0mC is at x=3.0m. Find the x-component, E, of ...
A:
Q: what is earth?
A: Earth is planet on which we live, and it is one of the 8 planet’s revolving around the sun. It is th...
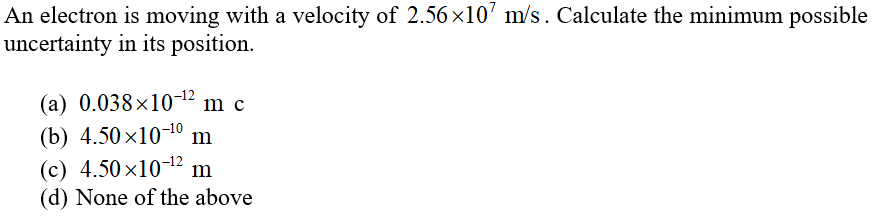
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images
