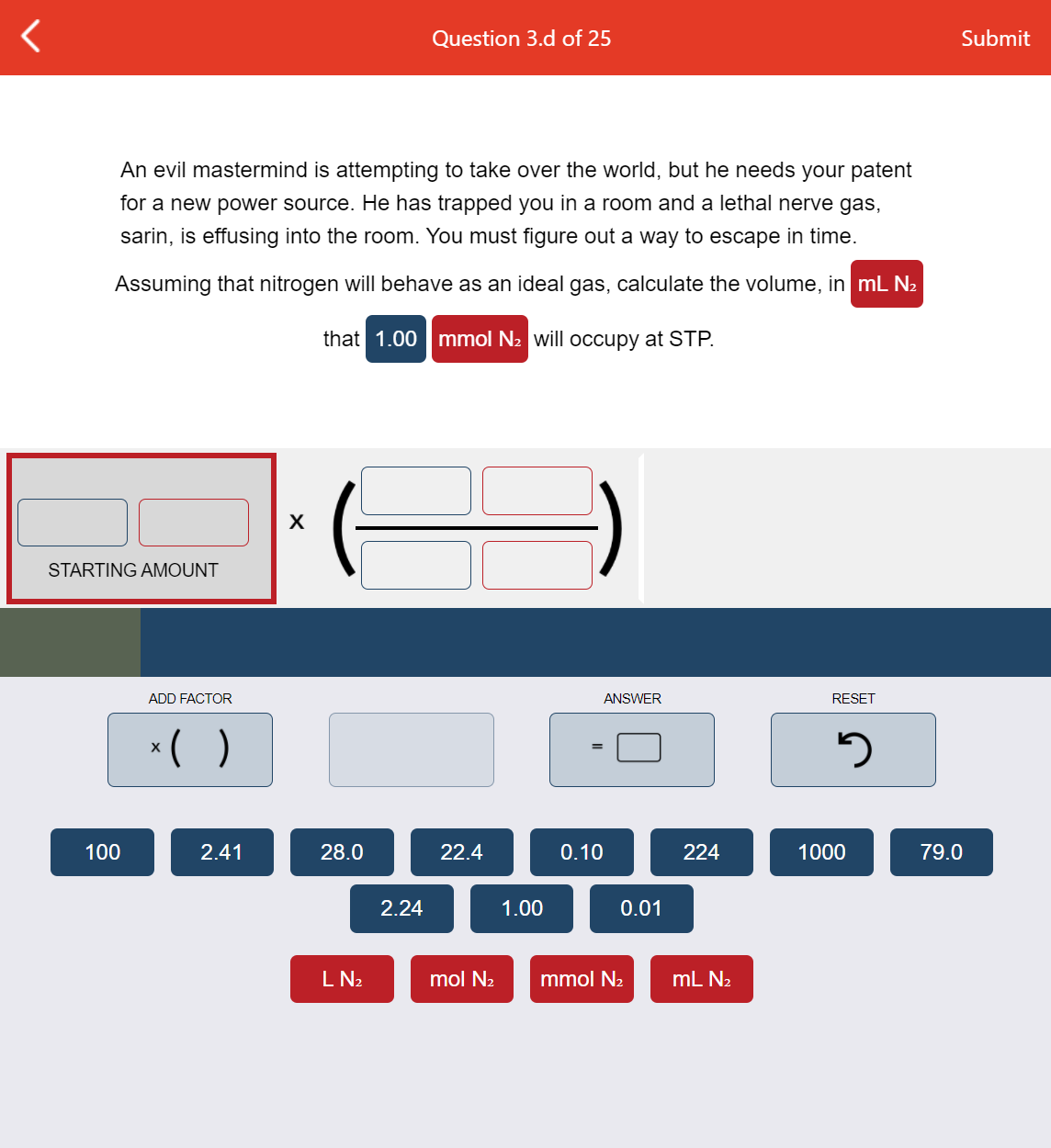
An evil mastermind is attempting to take over the world, but he needs your patent for a new power source. He has trapped you in a room and a lethal nerve gas, sarin, is effusing into the room. You must figure out a way to escape in time. Assuming that nitrogen will behave as an ideal gas, calculate the volume, in mL N2 that 1.00 mmol N2 will occupy at STP.
An evil mastermind is attempting to take over the world, but he needs your patent for a new power source. He has trapped you in a room and a lethal nerve gas, sarin, is effusing into the room. You must figure out a way to escape in time. Assuming that nitrogen will behave as an ideal gas, calculate the volume, in mL N2 that 1.00 mmol N2 will occupy at STP.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.14E
Related questions
Question
Transcribed Image Text:Question 3.d of 25
Submit
An evil mastermind is attempting to take over the world, but he needs your patent
for a new power source. He has trapped you in a room and a lethal nerve gas,
sarin, is effusing into the room. You must figure out a way to escape in time.
Assuming that nitrogen will behave as an ideal gas, calculate the volume, in mL N2
that 1.00 mmol N2 will occupy at STP.
X
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
100
2.41
28.0
22.4
0.10
224
1000
79.0
2.24
1.00
0.01
L N2
mol N2
mmol N2
mL N2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax