An intensive property is one which does vary with the amount of substance, such as volume. does not vary with the amount of substance, such as volume. does not vary with the amount of substance, such as boiling point. does vary with the amount of substance, such as boiling point. To the correct number of significant figures, what are the average and standard deviation of the following four numbers? You may use Excel to do your calculations. 7.159 7.024 7.183 7.005 Average Standard Deviation
An intensive property is one which does vary with the amount of substance, such as volume. does not vary with the amount of substance, such as volume. does not vary with the amount of substance, such as boiling point. does vary with the amount of substance, such as boiling point. To the correct number of significant figures, what are the average and standard deviation of the following four numbers? You may use Excel to do your calculations. 7.159 7.024 7.183 7.005 Average Standard Deviation
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter3: Matter
Section: Chapter Questions
Problem 7CR
Related questions
Question
Answer both questions with details solution
I'll give you ?
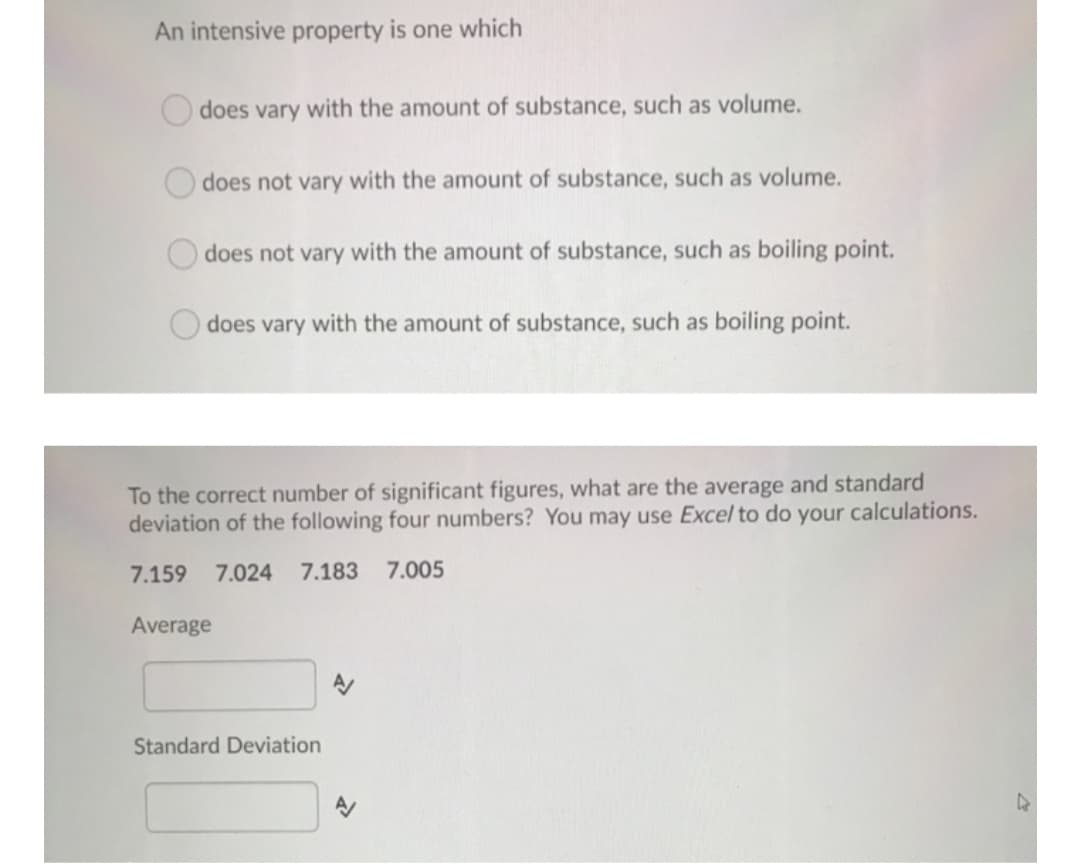
Transcribed Image Text:An intensive property is one which
does vary with the amount of substance, such as volume.
does not vary with the amount of substance, such as volume.
does not vary with the amount of substance, such as boiling point.
does vary with the amount of substance, such as boiling point.
To the correct number of significant figures, what are the average and standard
deviation of the following four numbers? You may use Excel to do your calculations.
7.159 7.024 7.183 7.005
Average
Standard Deviation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
