Answer Bank Close crucible and heat until reaction is complete. Set up support stand and heat source, and then add reactants and record the combined mass. Clean up and put away equipment. Remove the heat source and allow crucible and contents to cool completely. Wash and thoroughly dry crucible and cover. Weigh and record their combined mass when empty. Weigh crucible and lid with products.
Answer Bank Close crucible and heat until reaction is complete. Set up support stand and heat source, and then add reactants and record the combined mass. Clean up and put away equipment. Remove the heat source and allow crucible and contents to cool completely. Wash and thoroughly dry crucible and cover. Weigh and record their combined mass when empty. Weigh crucible and lid with products.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.56QE
Related questions
Question

Transcribed Image Text:A crucible and cover are made to withstand high temperatures that might damage other containers. When using a crucible
to heat a substance, a specific procedure should be followed.
Place the given steps in the correct order.
Start
Finish
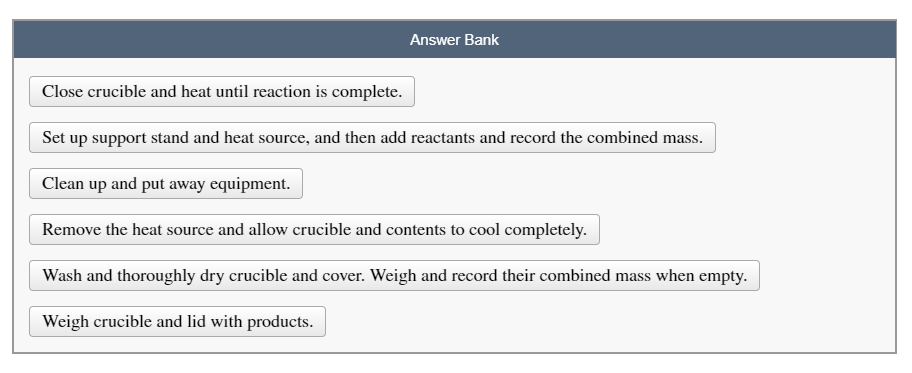
Transcribed Image Text:Answer Bank
Close crucible and heat until reaction is complete.
Set up support stand and heat source, and then add reactants and record the combined mass.
Clean up and put away equipment.
Remove the heat source and allow crucible and contents to cool completely.
Wash and thoroughly dry crucible and cover. Weigh and record their combined mass when empty.
Weigh crucible and lid with products.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div