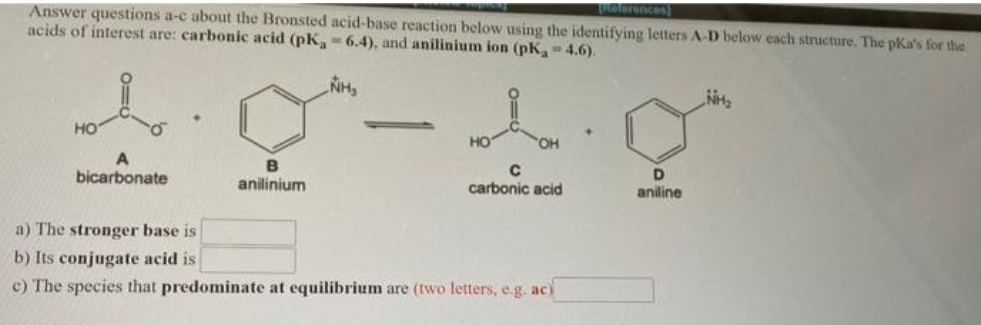
Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: carbonic acid (pK,-6.4), and anilinium ion (pK-4.6). NH HO OH D aniline bicarbonate anilinium carbonic acid a) The stronger base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.g. ac)
Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: carbonic acid (pK,-6.4), and anilinium ion (pK-4.6). NH HO OH D aniline bicarbonate anilinium carbonic acid a) The stronger base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.g. ac)
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter20: Acidity And Pka Of Phenols
Section: Chapter Questions
Problem 5E
Related questions
Question
Transcribed Image Text:TRolerences)
Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the
acids of interest are: carbonic acid (pK,-6.4), and anilinium ion (pK-4.6).
NH
но
HO
OH
B
D.
bicarbonate
anilinium
carbonic acid
aniline
a) The stronger base is
b) Its conjugate acid is
c) The species that predominate at equilibrium are (two letters, e.g. ac)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
