Answer the following questions, modelling air as a diatomic ideal gas with average molar mass M = 29 g/mol and the ratio of specific heats y Cp = 1.4. Cy 1. Show that the pressure gradient for the change of altitude is given by dP MgP dy RT where y is the altitude from the ground, g is the gravitational acceleration which is assumed to be constant, T is the temperature and R is the gas constant.
Answer the following questions, modelling air as a diatomic ideal gas with average molar mass M = 29 g/mol and the ratio of specific heats y Cp = 1.4. Cy 1. Show that the pressure gradient for the change of altitude is given by dP MgP dy RT where y is the altitude from the ground, g is the gravitational acceleration which is assumed to be constant, T is the temperature and R is the gas constant.
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter10: Statistical Physics
Section: Chapter Questions
Problem 8P
Related questions
Question
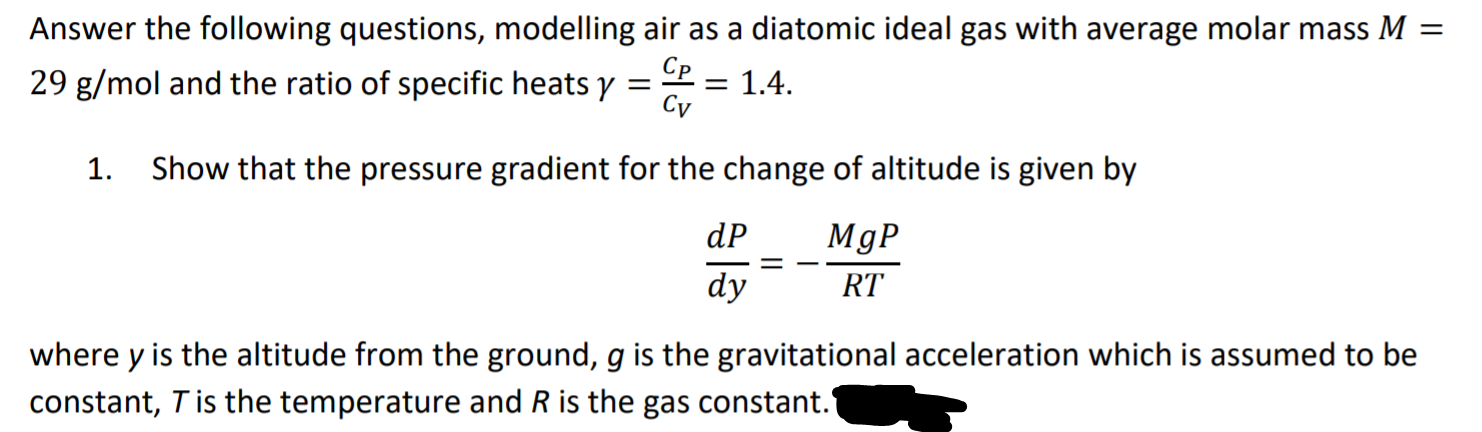
Transcribed Image Text:Answer the following questions, modelling air as a diatomic ideal gas with average molar mass M =
29 g/mol and the ratio of specific heats y
Cp
= 1.4.
Cy
1. Show that the pressure gradient for the change of altitude is given by
dP
MgP
dy
RT
where y is the altitude from the ground, g is the gravitational acceleration which is assumed to be
constant, T is the temperature and R is the gas constant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning