Answer the following questions using the diagram shown below. Free Energy Reactants Reaction Coordinate a) Elementary step 1: A+ B➜ C Elementary step 2: CD+ E Elementary step 3: D + E➜ Products Transition state b) Elementary step 1: A+ BC Elementary step 2: CD Elementary step 3: DF Elementary step 4: F→ Products Reactive intermediate 1. Select the correct hypothetical reaction scheme from below (a and b) that can be represented by the graph above. Given, both reactions start from the same reagents and give the same products but uses different elementary steps. Products 1 Provide an explanation for your selection above. Make sure to use the graph provided to justify your answer. Is the overall reaction shown above spontaneous/non-spontaneous? Justify your answer.
Answer the following questions using the diagram shown below. Free Energy Reactants Reaction Coordinate a) Elementary step 1: A+ B➜ C Elementary step 2: CD+ E Elementary step 3: D + E➜ Products Transition state b) Elementary step 1: A+ BC Elementary step 2: CD Elementary step 3: DF Elementary step 4: F→ Products Reactive intermediate 1. Select the correct hypothetical reaction scheme from below (a and b) that can be represented by the graph above. Given, both reactions start from the same reagents and give the same products but uses different elementary steps. Products 1 Provide an explanation for your selection above. Make sure to use the graph provided to justify your answer. Is the overall reaction shown above spontaneous/non-spontaneous? Justify your answer.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter18: Chemical Kinetics
Section: Chapter Questions
Problem 43P
Related questions
Question
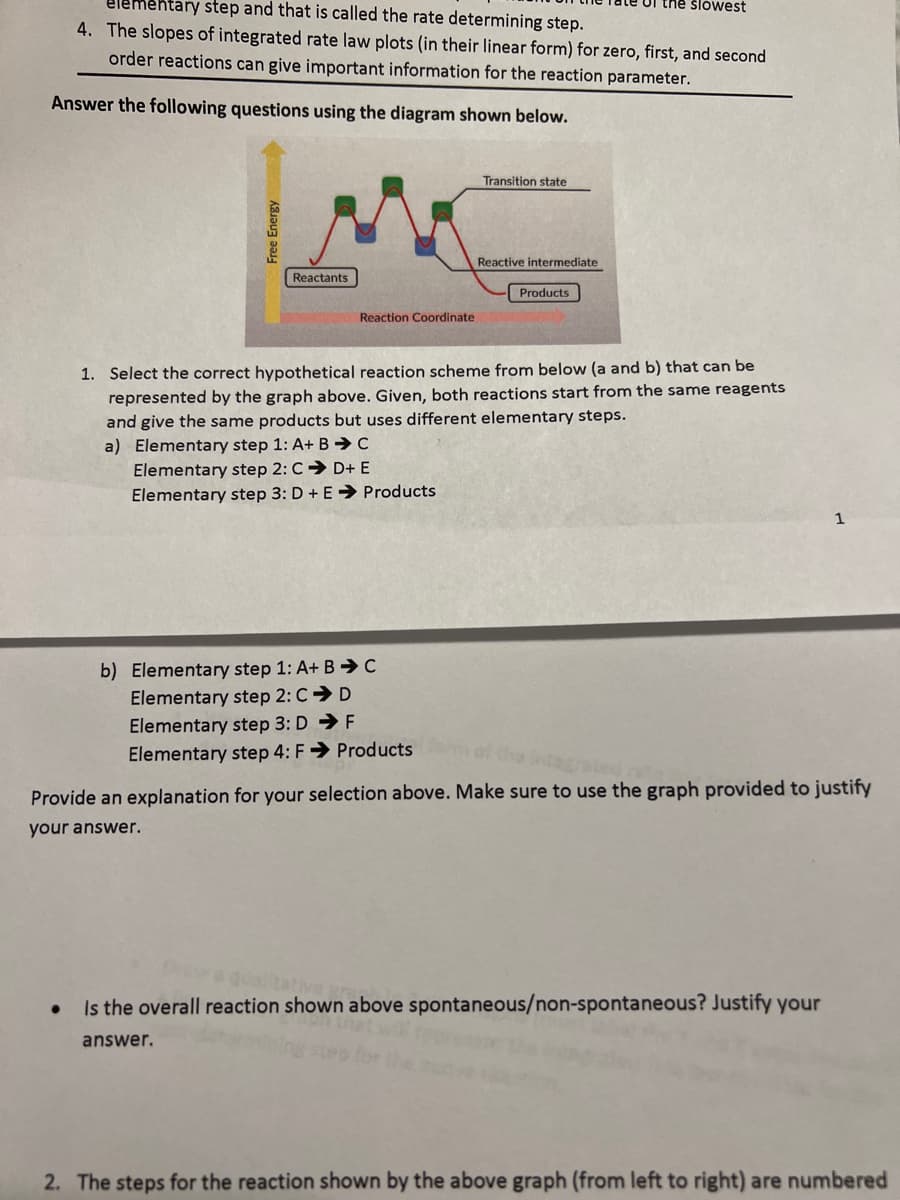
Transcribed Image Text:elementary step and that is called the rate determining step.
4. The slopes of integrated rate law plots (in their linear form) for zero, first, and second
order reactions can give important information for the reaction parameter.
Answer the following questions using the diagram shown below.
Free Energy
●
Reactants
Reaction Coordinate
a) Elementary step 1: A+ B C
Elementary step 2: CD+ E
Elementary step 3: D + E➜ Products
Transition state
b) Elementary step 1: A+ BC
Elementary step 2: CD
Elementary step 3: DF
Elementary step 4: F→ Products
Reactive intermediate
Products
1. Select the correct hypothetical reaction scheme from below (a and b) that can be
represented by the graph above. Given, both reactions start from the same reagents
and give the same products but uses different elementary steps.
the slowest
Provide an explanation for your selection above. Make sure to use the graph provided to justify
your answer.
1
Is the overall reaction shown above spontaneous/non-spontaneous? Justify your
answer.
2. The steps for the reaction shown by the above graph (from left to right) are numbered
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning