APPENDIX C Ihermodynamic Quantities for Selected Substances at 298.15 K (25 1091 "C) AG (ki/mal) дн; (ki/mol) AGi (kj/mol) S' (kj/mol) -245.6 (1/mol-K) (i/mol-K) Substance Vanadium Substance SOCIE(1) HS(8) HSO4(aq) HSO,() -20.17 -33.01 205.6 453.1 182.2 514.2 Vg) V(s) - 744.5 20.1 156.1 -909.3 28.9 -814.0 -689.9 Zinc Titanium 130.7 95.2 160.9 Zn(g) TIg) TI(S) TICL() TICL(1) TIO:(s) 468 422 180.3 30.76 354.9 41.63 Zn(s) ZnCl, (8) ZnO(s) -369.4 -415.1 111.5 -763.2 -726.8 -728.1 -889.4 -348.0 -318.2 43.9 -804.2 -944.7 221.9 50.29 Average Bond Enthalpies (kJ/mol) TABLE 8.3 Single Bonds C-H 413 N-H 391 О—н 463 F-F 155 348 N-N 163 0-0 146 CI-F 293 201 0-F 190 253 C-0 358 N-F 272 0-CI 203 Cl-CI 242 C-F 485 200 0-I 234 N-CI 243 C-CI 328 N-Br Br-F 237 C-Br 276 S-H 339 Br-CI 218 C-I 240 Н-Н 436 S-F 327 Br-Br 193 C-S 259 Н—F 567 S-CI 253 Н—СІ 431 S-Br 218 I-CI 208 Si-H 323 Н—Br 366 S-S 266 I-Br 175 Si-Si 226 Н-I 299 I-I 151 Si-C 301 Si-O 368 Si-CI 464 Multiple Bonds 495 614 N=N 418 0=0 839 N=N 941 615 607 523 C=N 891 S=S 418 799 1072
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Acetylene (C2H2) and nitrogen (N2) both contain a triple
bond, but they differ greatly in their chemical properties.
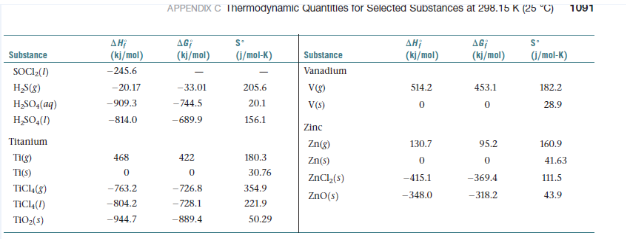
(a) Write the Lewis structures for the two substances. (b) By referring to Appendix C, look up the enthalpies of formation
of acetylene and nitrogen. Which compound is more stable?
(c) Write balanced chemical equations for the complete
oxidation of N2 to form N2O5(g) and of acetylene to form
CO2(g) and H2O(g). (d) Calculate the enthalpy of oxidation
per mole for N2 and for C2H2 (the enthalpy of formation
of N2O5(g) is 11.30 kJ/mol). (e) Both N2 and C2H2 possess
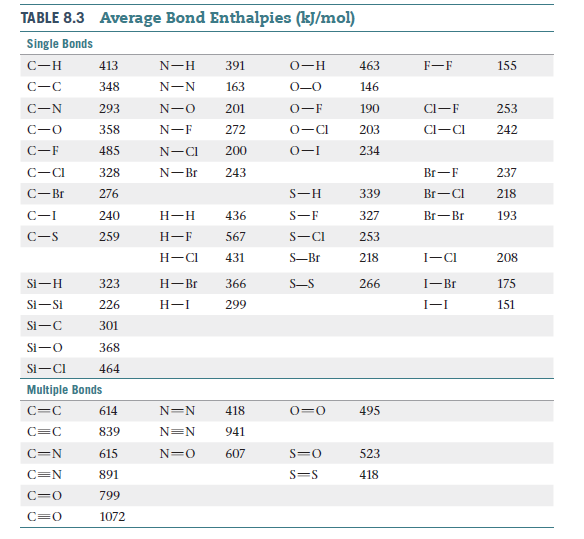
triple bonds with quite high bond enthalpies (Table 8.3).
Calculate the enthalpy of hydrogenation per mole for both
compounds: acetylene plus H2 to make methane, CH4;
nitrogen plus H2 to make ammonia, NH3.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images








