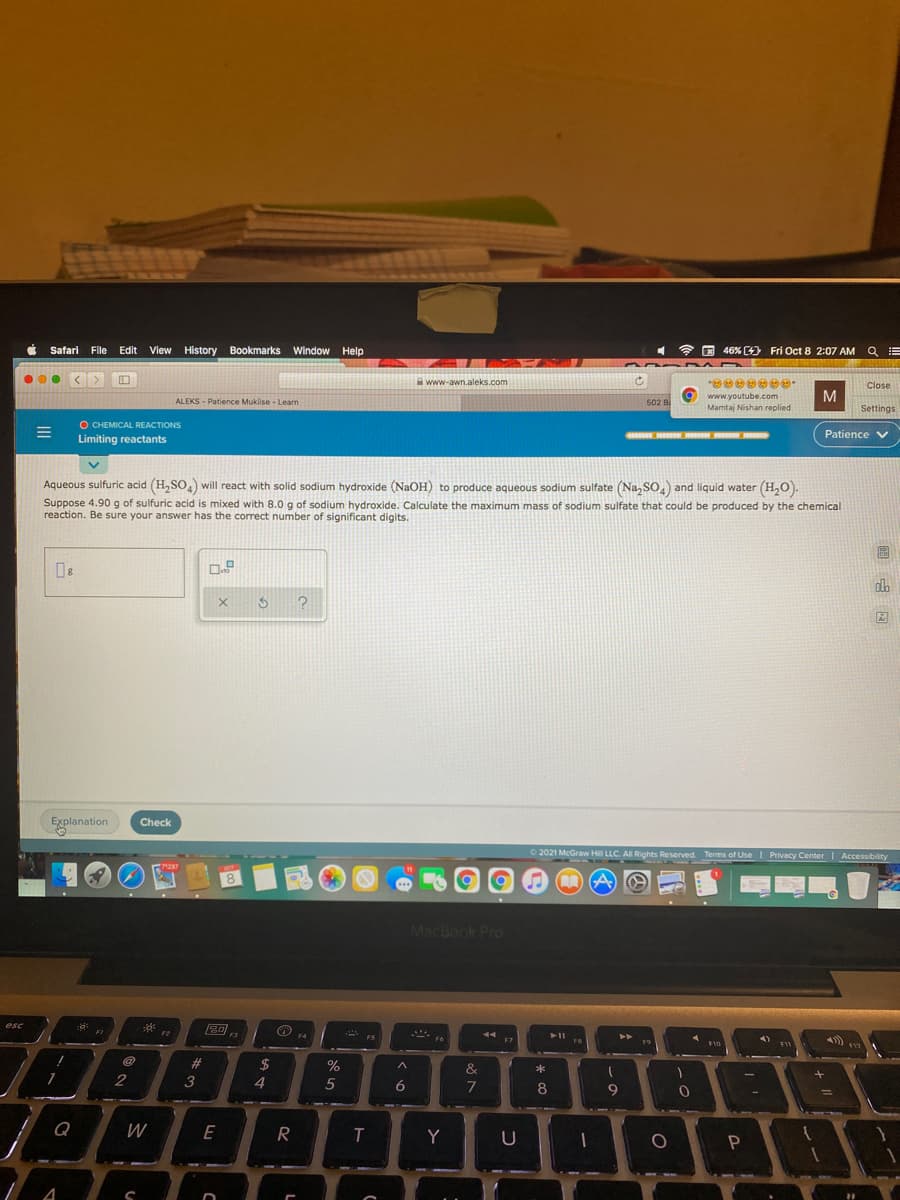
Aqueous sulfuric acid (H,SO) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, so.) and liquid water (H,O). Suppose 4.90 g of sulfuric acid is mixed with 8.0 g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. do
Aqueous sulfuric acid (H,SO) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, so.) and liquid water (H,O). Suppose 4.90 g of sulfuric acid is mixed with 8.0 g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. do
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 52QAP: Write a balanced equation for the reaction between (a) dihydrogen sulfide and sulfur dioxide gases...
Related questions
Question
100%
Transcribed Image Text:Safari File
Edit View History Bookmarks Window Help
A E
46% (4) Fri Oct 8 2:07 AM QE
Awww-awn.aleks.com
Close
M
www.youtube.com
Mamtaj Nishan replied
ALEKS - Patience Muklise - Learn
502 B
Settings
O CHEMICAL REACTIONS
Limiting reactants
Patience v
Aqueous sulfuric acid (H,SO,) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium sulfate (Na, SO,) and liquid water (H,O).
Suppose 4.90g of sulfuric acid is mixed with 8.0 g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical
reaction. Be sure your answer has the correct number of significant digits.
dlo
Explanation
Check
O 2021 McGraw Hl LLC. All Rights Reserved. Terms of Use I Privacy Center | Accessibility
171237
DCT
8.
MacBook Pro
esc
F2
F3
F6
F7
F9
F10
FII
F12
%23
2$
&
*
4
5
7
8
Q
W
R
Y
U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning