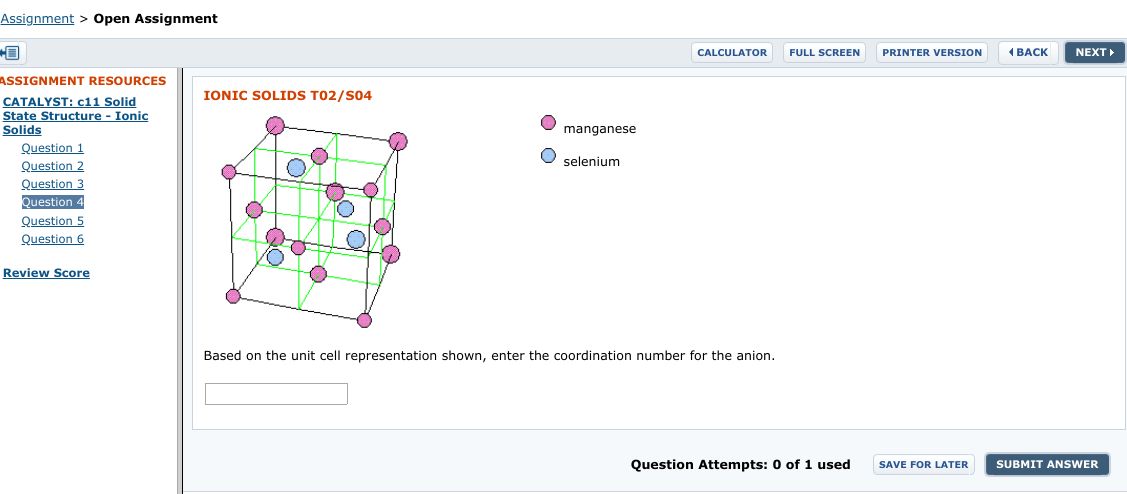
Assignment > Open Assignment ( BACK PRINTER VERSION NEXT CALCULATOR FULL SCREEN ASSIGNMENT RESOURCES IONIC SOLIDS T02/SO4 CATALYST: c11 Solid State Structure - Ionic Solids manganese Question 1 selenium Question 2 Question 3 Question 4 Question 5 Question 6 Review Score Based on the unit cell representation shown, enter the coordination number for the anion. Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
Assignment > Open Assignment ( BACK PRINTER VERSION NEXT CALCULATOR FULL SCREEN ASSIGNMENT RESOURCES IONIC SOLIDS T02/SO4 CATALYST: c11 Solid State Structure - Ionic Solids manganese Question 1 selenium Question 2 Question 3 Question 4 Question 5 Question 6 Review Score Based on the unit cell representation shown, enter the coordination number for the anion. Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 57AP
Related questions
Question
Please help me and double and triple check your answers my grade depends on this
Transcribed Image Text:Assignment > Open Assignment
( BACK
PRINTER VERSION
NEXT
CALCULATOR
FULL SCREEN
ASSIGNMENT RESOURCES
IONIC SOLIDS T02/SO4
CATALYST: c11 Solid
State Structure - Ionic
Solids
manganese
Question 1
selenium
Question 2
Question 3
Question 4
Question 5
Question 6
Review Score
Based on the unit cell representation shown, enter the coordination number for the anion.
Question Attempts: 0 of 1 used
SUBMIT ANSWER
SAVE FOR LATER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,