Assuming equal concentrations, arrange these solutions by pH Highest pH NH, (aq) RBOH(aq) Ca(OH)2 (aq) HF(aq) HBr(aq) Lowest pH Answer Bank help about us terms of use privacy policy contact us careers ENC 12 hp
Assuming equal concentrations, arrange these solutions by pH Highest pH NH, (aq) RBOH(aq) Ca(OH)2 (aq) HF(aq) HBr(aq) Lowest pH Answer Bank help about us terms of use privacy policy contact us careers ENC 12 hp
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.53QE
Related questions
Question
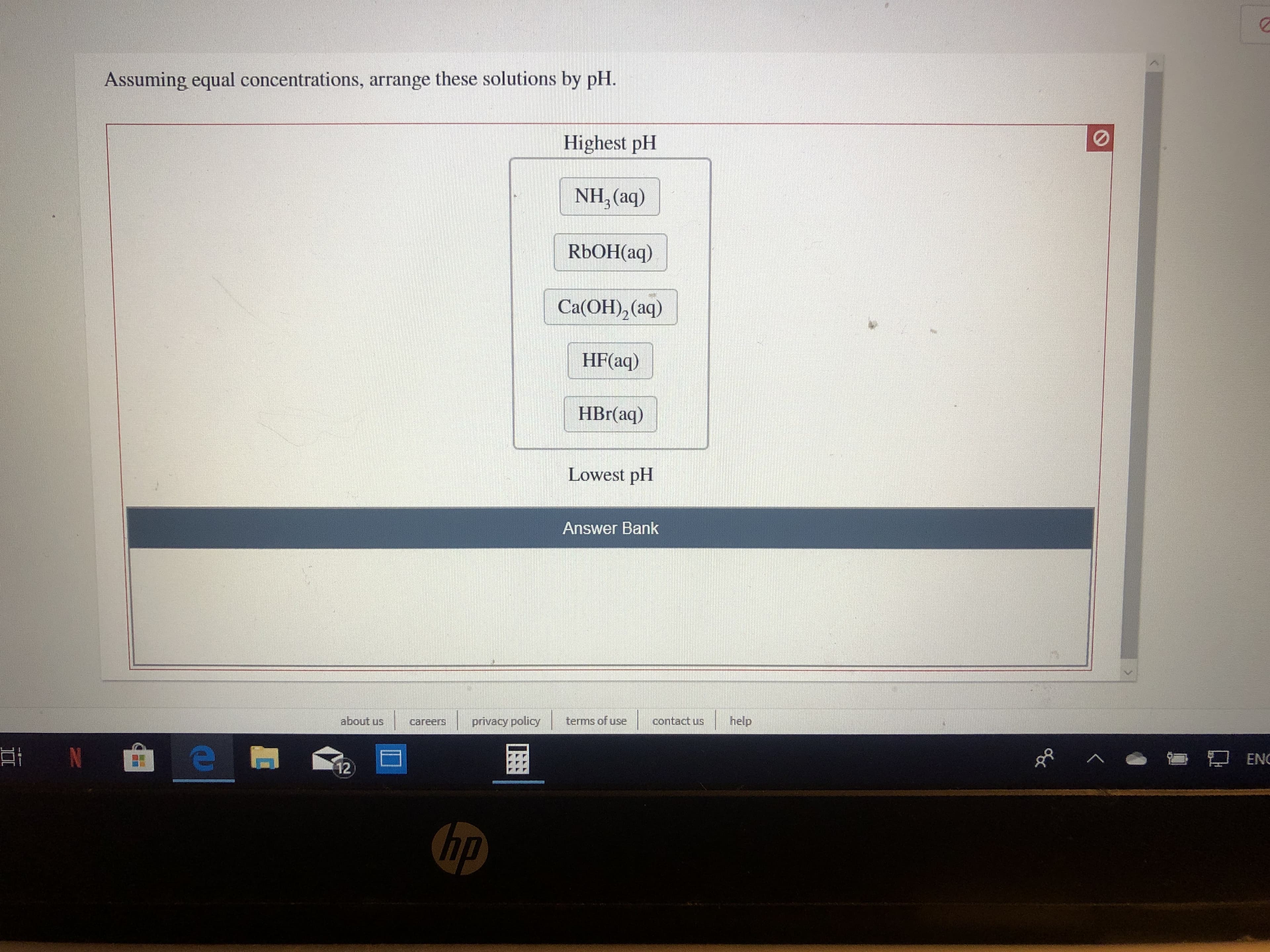
Transcribed Image Text:Assuming equal concentrations, arrange these solutions by pH
Highest pH
NH, (aq)
RBOH(aq)
Ca(OH)2 (aq)
HF(aq)
HBr(aq)
Lowest pH
Answer Bank
help
about us
terms of use
privacy policy
contact us
careers
ENC
12
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning