At 298 K, the equilibrium constant for the below reaction is 4.17 x 103. What is the concentration of Cl at equilibrium? 6. Pb2+ (aq)2 cl- (aq) = PbCl2 (s) (а) 20.2 М (с) 10.1 М (b) 0.078 M (d) 0.039 M
At 298 K, the equilibrium constant for the below reaction is 4.17 x 103. What is the concentration of Cl at equilibrium? 6. Pb2+ (aq)2 cl- (aq) = PbCl2 (s) (а) 20.2 М (с) 10.1 М (b) 0.078 M (d) 0.039 M
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 9.76EP: Calculate the value of the equilibrium constant for the reaction N2(g)+2O2(g)2NO2(g) if the...
Related questions
Question
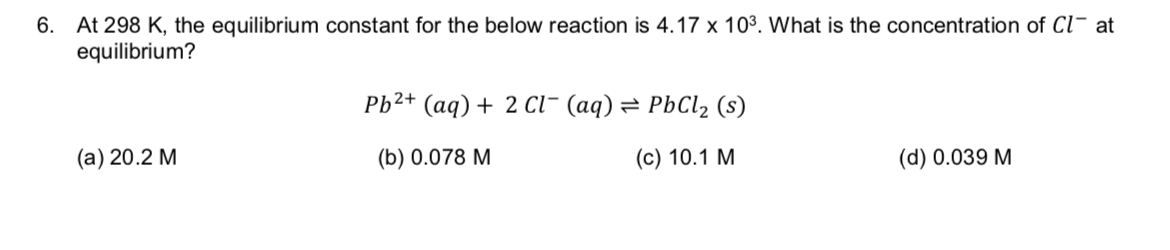
Transcribed Image Text:At 298 K, the equilibrium constant for the below reaction is 4.17 x 103. What is the concentration of Cl at
equilibrium?
6.
Pb2+ (aq)2 cl- (aq) = PbCl2 (s)
(а) 20.2 М
(с) 10.1 М
(b) 0.078 M
(d) 0.039 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax