At a certain temperature, the equilibrium constant K for the following reaction is 0.0085: C1, (g) + CHC1, (g)- HC1(g) + CC1,(g) Use this information to complete the following table. Suppose a 41. L reaction vessel is filled with 1.9 mol of Clz and 1.9 mol of CHCI3. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little Cl, and CHCI3. There vwill be very little HCl and Ccl. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = | HCl(9)+CC1, (9) C1,(9)+ CHC1;(9) 1, What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 C1,(9)+2CHC1,(9) 2 HCl(9)+2CC1,(9) 1,
At a certain temperature, the equilibrium constant K for the following reaction is 0.0085: C1, (g) + CHC1, (g)- HC1(g) + CC1,(g) Use this information to complete the following table. Suppose a 41. L reaction vessel is filled with 1.9 mol of Clz and 1.9 mol of CHCI3. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little Cl, and CHCI3. There vwill be very little HCl and Ccl. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = | HCl(9)+CC1, (9) C1,(9)+ CHC1;(9) 1, What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 C1,(9)+2CHC1,(9) 2 HCl(9)+2CC1,(9) 1,
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 61QRT
Related questions
Question
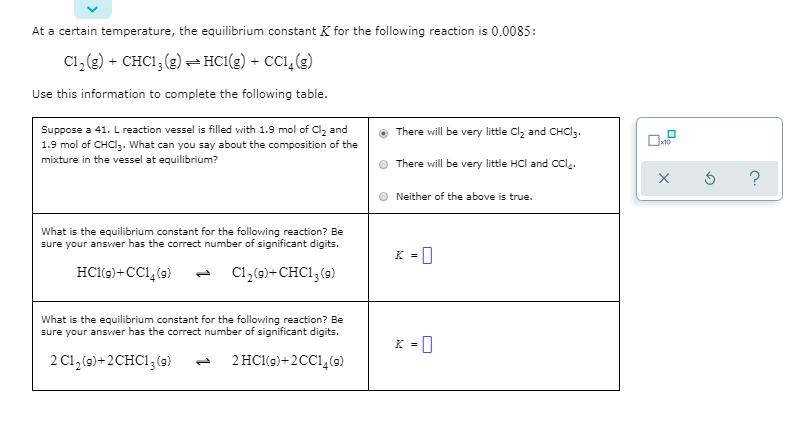
Transcribed Image Text:At a certain temperature, the equilibrium constant K for the following reaction is 0.0085:
C1, (g) + CHC1, (g)- HC1(g) + CC1,(g)
Use this information to complete the following table.
Suppose a 41. L reaction vessel is filled with 1.9 mol of Clz and
1.9 mol of CHCI3. What can you say about the composition of the
mixture in the vessel at equilibrium?
There will be very little Cl, and CHCI3.
There vwill be very little HCl and Ccl.
Neither of the above is true.
What is the equilibrium constant for the following reaction? Be
sure your answer has the correct number of significant digits.
K = |
HCl(9)+CC1, (9)
C1,(9)+ CHC1;(9)
1,
What is the equilibrium constant for the following reaction? Be
sure your answer has the correct number of significant digits.
2 C1,(9)+2CHC1,(9)
2 HCl(9)+2CC1,(9)
1,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning