(A)The following figure is the structure of Glycerin molecule. There is a certain functional group present in this molecule. Answer the following questions about this molecule. CH2OH H- HO- ČH2OH a) Name the functional group that is present in the molecule? b) Calculate the total number of bonds present in this molecule. B) A standard solution of glucose of volume 1 liters having Molarity 1M was prepared in the chemistry laboratory. Later, this solution was diluted to change its concentration to 0.5M. How much water was needed to bring about this change?
(A)The following figure is the structure of Glycerin molecule. There is a certain functional group present in this molecule. Answer the following questions about this molecule. CH2OH H- HO- ČH2OH a) Name the functional group that is present in the molecule? b) Calculate the total number of bonds present in this molecule. B) A standard solution of glucose of volume 1 liters having Molarity 1M was prepared in the chemistry laboratory. Later, this solution was diluted to change its concentration to 0.5M. How much water was needed to bring about this change?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 93AP
Related questions
Question
100%
8- Please I want answer with steps. Many thanks
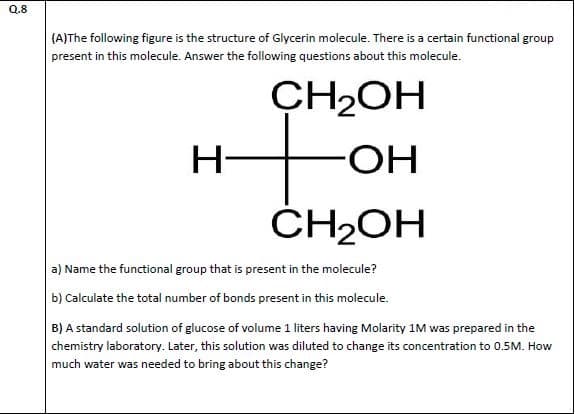
Transcribed Image Text:(A)The following figure is the structure of Glycerin molecule. There is a certain functional group
present in this molecule. Answer the following questions about this molecule.
CH2OH
H-
HO-
ČH2OH
a) Name the functional group that is present in the molecule?
b) Calculate the total number of bonds present in this molecule.
B) A standard solution of glucose of volume 1 liters having Molarity 1M was prepared in the
chemistry laboratory. Later, this solution was diluted to change its concentration to 0.5M. How
much water was needed to bring about this change?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning