b A large beaker contains 1.50 L of X M Your Chemistry answer is ready G mass per litre equation - Google WS9-Trade.docx LON-CAPA Homework 11-11 X X C access1.lon-capa.uiuc.edu/res/uiuc/marville/chem102/102HWproblems/common_ion_effect.problem?symb=uploaded%2fuiuc%2f63206040cd3285d3euiuclibrary1%2fdefault_11508214... CHEM 102B/C Fall 2019 Lorcan James McGrath (Student - section: CQB) Messages Courses Help Logout Main Menu Contents Grades Course Contents » ... » Homework 11 » Homework 11-11 Timer Calculate the mass per liter of solid lead (II) phosphate (Ken = 1.00 x 10-54) that should dissolve in 0.450 M lead (II) nitrate solution. Incorrect. Tries 2/99 Previous Tries Submit Answer Send Feedback Post Discussion B sky е вы Bb f 1 16:41 sports .. o
b A large beaker contains 1.50 L of X M Your Chemistry answer is ready G mass per litre equation - Google WS9-Trade.docx LON-CAPA Homework 11-11 X X C access1.lon-capa.uiuc.edu/res/uiuc/marville/chem102/102HWproblems/common_ion_effect.problem?symb=uploaded%2fuiuc%2f63206040cd3285d3euiuclibrary1%2fdefault_11508214... CHEM 102B/C Fall 2019 Lorcan James McGrath (Student - section: CQB) Messages Courses Help Logout Main Menu Contents Grades Course Contents » ... » Homework 11 » Homework 11-11 Timer Calculate the mass per liter of solid lead (II) phosphate (Ken = 1.00 x 10-54) that should dissolve in 0.450 M lead (II) nitrate solution. Incorrect. Tries 2/99 Previous Tries Submit Answer Send Feedback Post Discussion B sky е вы Bb f 1 16:41 sports .. o
Chapter15: Electrophoresis And Other Separation Methods
Section: Chapter Questions
Problem 1P
Related questions
Question
Transcribed Image Text:b A large beaker contains 1.50 L of X M Your Chemistry answer is ready
G mass per litre equation - Google
WS9-Trade.docx
LON-CAPA Homework 11-11
X
X
C
access1.lon-capa.uiuc.edu/res/uiuc/marville/chem102/102HWproblems/common_ion_effect.problem?symb=uploaded%2fuiuc%2f63206040cd3285d3euiuclibrary1%2fdefault_11508214...
CHEM 102B/C Fall 2019
Lorcan James McGrath (Student - section: CQB)
Messages Courses Help Logout
Main Menu
Contents
Grades
Course Contents » ... » Homework 11 » Homework 11-11
Timer
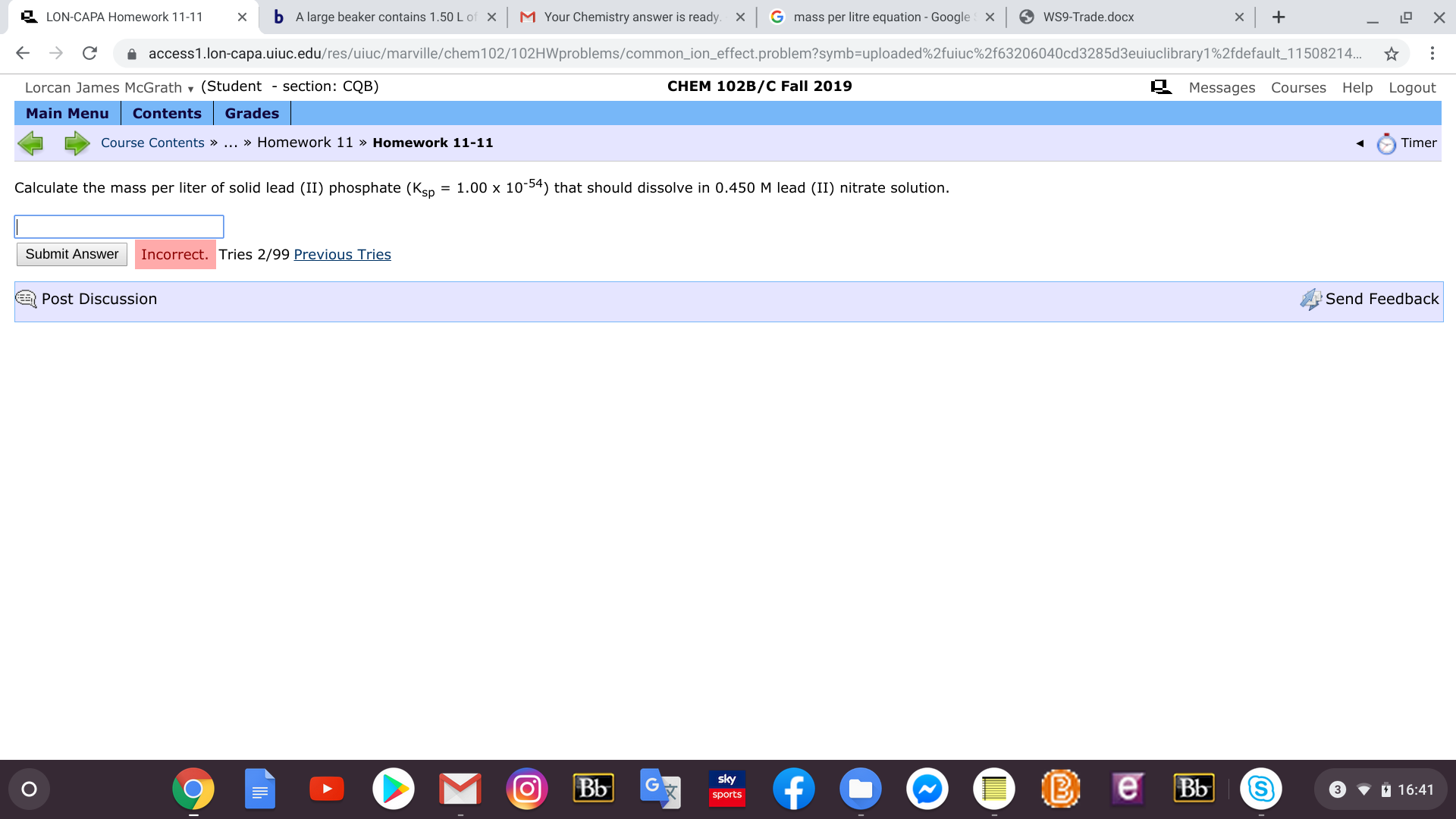
Calculate the mass per liter of solid lead (II) phosphate (Ken = 1.00 x 10-54) that should dissolve in 0.450 M lead (II) nitrate solution.
Incorrect. Tries 2/99 Previous Tries
Submit Answer
Send Feedback
Post Discussion
B
sky
е вы
Bb
f
1 16:41
sports
..
o
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

