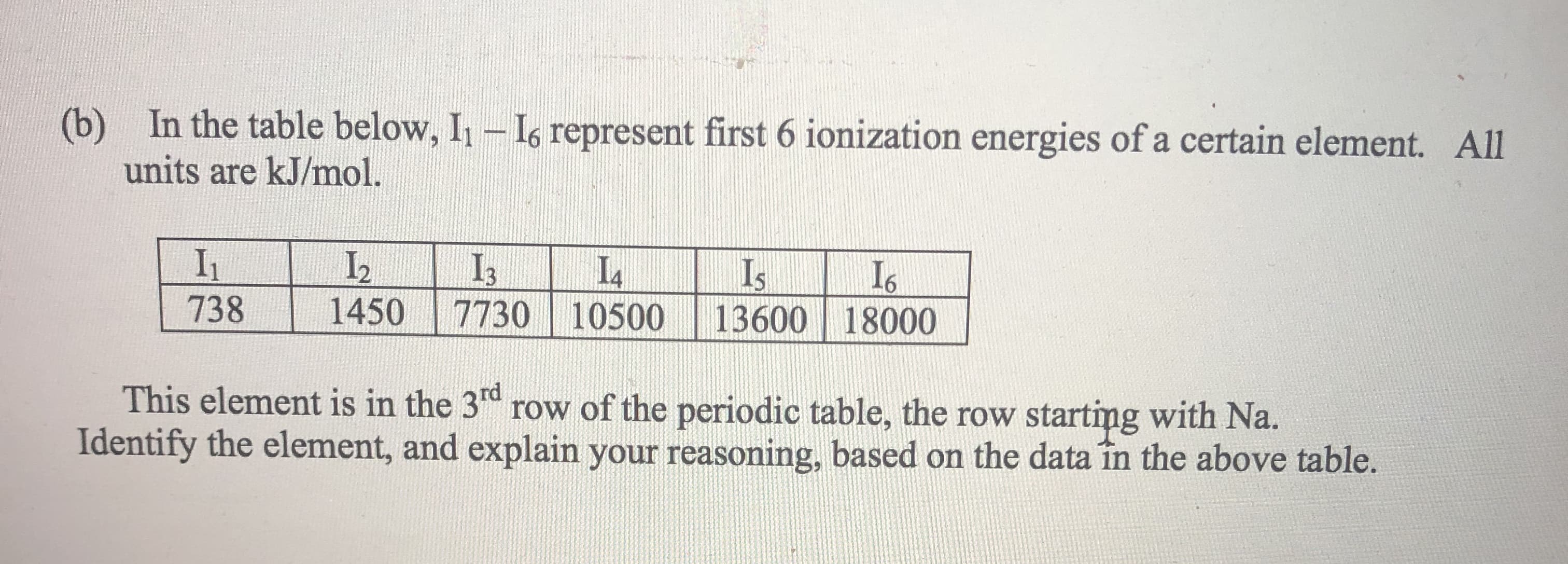
(b) In the table below, I- Is represent first 6 ionization energies of a certain element. All units are kJ/mol. I1 738 L2 1450 I3 I4 Is 13600 18000 7730 10500 This element is in the 3d row of the periodic table, the row Identify the element, and explain your reasoning, based on the data în the above table. starting with Na.
(b) In the table below, I- Is represent first 6 ionization energies of a certain element. All units are kJ/mol. I1 738 L2 1450 I3 I4 Is 13600 18000 7730 10500 This element is in the 3d row of the periodic table, the row Identify the element, and explain your reasoning, based on the data în the above table. starting with Na.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter8: The Periodic Table: Structure And Trends
Section: Chapter Questions
Problem 8.9QE
Related questions
Question
In the table below, I1 – I6 represent first 6 ionization energies of a certain element. All units are kJ/mol.
I1 I2 I3 I4 I5 I6
738 1450 7730 10500 13600 18000
This element is in the 3rd row of the periodic table, the row starting with Na. Identify the element, and explain your reasoning, based on the data in the above table.
Transcribed Image Text:(b) In the table below, I- Is represent first 6 ionization energies of a certain element. All
units are kJ/mol.
I1
738
L2
1450
I3
I4
Is
13600 18000
7730 10500
This element is in the 3d row of the periodic table, the row
Identify the element, and explain your reasoning, based on the data în the above table.
starting with Na.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning