(b) Photoelectron spectroscopy data for the 1s sublevel of Ne and the 1s sublevel of Xe are represented below. In terms of Coulomb's law and atomic structure, explain why the peak for Ne is positioned so far to the right of the peak for Xe. Ne 3500 3000 2500 2000 1500 1000 500 Binding Energy (MJ/mol) Хе 3500 3000 2500 2000 1500 1000 500 Binding Energy (MJ/mol) Relative Number of Electrons Relative Number of Electrons
(b) Photoelectron spectroscopy data for the 1s sublevel of Ne and the 1s sublevel of Xe are represented below. In terms of Coulomb's law and atomic structure, explain why the peak for Ne is positioned so far to the right of the peak for Xe. Ne 3500 3000 2500 2000 1500 1000 500 Binding Energy (MJ/mol) Хе 3500 3000 2500 2000 1500 1000 500 Binding Energy (MJ/mol) Relative Number of Electrons Relative Number of Electrons
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.3PAE
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
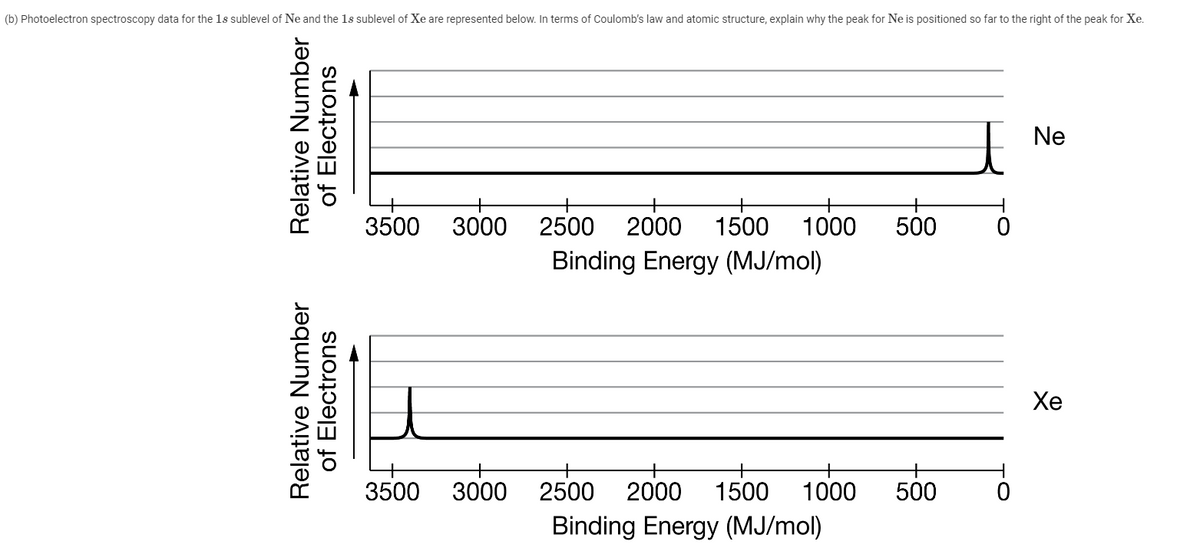
Transcribed Image Text:(b) Photoelectron spectroscopy data for the 1s sublevel of Ne and the 1s sublevel of Xe are represented below. In terms of Coulomb's law and atomic structure, explain why the peak for Ne is positioned so far to the right of the peak for Xe.
Ne
3500
3000
2500 2000 1500
1000
500
Binding Energy (MJ/mol)
Хе
3500
3000
2500
2000
1500
1000
500
Binding Energy (MJ/mol)
Relative Number
Relative Number
of Electrons
of Electrons
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Expert Answers to Latest Homework Questions
Q: At Blossom Electronics, it costs $33 per unit ($15 variable and $18 fixed) to make an MP3 player…
Q: Cullumber Company is considering two alternatives. Alternative A will have revenues of $147,300 and…
Q: Initially , volume in the ball was 750 mL when it was filled with 2.5 moles of gas. What will be…
Q: There is 16.5 kg of silver at 19 degrees Celsius. How much heat is needed to melt it? (Melting point…
Q: If there is a container of liquid oxygen at -183 degrees Celsius, how much of it can evaporate if…
Q: What volume ( in Liters) will 2.0 g of hydrogen gas occupy at STP conditions?
Q: Consider the linear regression models. The p-values are shown in parenthesis.Model 1 Y = 123.2 +…
Q: What volume ( in Liters) will 2.0 g of hydrogen gas occupy at STP conditions?
Q: A 0.40 kg iron hook is forged and dropped into 1.25 L of water in a 0.31 kg iron pot at 20 degrees…
Q: Kinetic Molecular Theory (KMT) of gases was developed in the late 19th century.
One of the main…
Q: Gases can be mixed with any proportions unless they chemically react with one another).
We can say…
Q: The molar mass of hydrogen (H) is 1 g/mol, the molar mass of chlorine (CI) is 35
g/mol, and the…
Q: Iron combines with oxygen to produce rust according to the following reaction.
Based on the law of…
Q: In the reaction, sodium and chlorine combine to produce sodium chloride. In an
instance when four…
Q: The value of specific heat for copper is 390 J/kgC for aluminum is 900 J/kgC and for water is 4186…
Q: Which statement about the equation is correct?
FeCl3 + 3NH4OH → Fe(OH)3 + 3NH4CI
О
Mass is conserved…
Q: A student claims that the reaction of hydrogen and oxygen to form hydrogen
peroxide is evidence…
Q: Calculate the value of is for H+, He+ and Li+2 ions at r = 0, r = a, and r = 4a,, Rationalize the…
Q: What is the specific heat of a substance if 120 kJ of heat is needed to raise 4.1 kg of the…
Q: Write the ground state electron configurations for the d-metals from Y to Cd. (Shorthand notation is…
Q: Explain why the 2s and 2p energy levels are at the same energy for Li+2.