b. A cell constructed with a Fe | Fe2 half-cell and a Pb | Pb2+ half cell 1. Determine Ecell (V) 2. Write the net ionic equation for the cell. Work Problem 3. Identify the half-cell at the cathode (enter a number 1 - 4) 1. Fe2+ (aq)+2 e 2. Fe(s)Fe2+ 3. Pb2+ Fes) (aq) +2 e (aq)+2 e Pb(s) 4. Pb/sPb2+ 2 e (aq) 2. Use a table of Standard Electron Reduction Potentials (click the Reference Materials link in the header of this webpage and then the Standard Reduction Potentials link) to determine the E° for the following standard cells. Write the net ionic equation for the cell. Identify which half-cell will be the cathode. Directions For Writing Molecular, lonic, And Net lonic Equations: 1. Molecular Equation: balance the molecular equation by predicting the products and changing the coefficients so that all atoms are balanced. Equations must be balanced to obey the Law of Conservation of Matter 2. lonic Equation: the ionic equation is obtained by breaking apart into ions any strong acid, strong base or soluble salt that has an (ag) after its formula - the ion's new coefficient will equal the coefficient (from the balanced equation) TIMES the ion's subscript (i.e. 2 Na2SO4 becomes 4 Na* and 2 SO42-). 3. Do Not Break Apart any reactant or product that has an (s) or () or (g) after its formula - list it exactly as it appears in the molecular equation 4. Do Not Break Apart a weak acid or base that has an (aq) after its formula - by definition a weak acid (or base) is not 100% ionized so it is represented as a molecule and not as ions. 5. Net lonic Equation: write the net ionic equation by cancelling out any spectator ions (ions that appear on both sides of the equation) and simplifying the coefficients to the smallest whole number ratio (if necessary). 6. Make sure to include the charge and state for the ions and molecules - all molecules have a charge of zero. 7.As a final check, the total charge on the reactant side must equal the total charge on the product side - this fact will aid you in writing Red Ox (Reduction Oxidation) reactions.
b. A cell constructed with a Fe | Fe2 half-cell and a Pb | Pb2+ half cell 1. Determine Ecell (V) 2. Write the net ionic equation for the cell. Work Problem 3. Identify the half-cell at the cathode (enter a number 1 - 4) 1. Fe2+ (aq)+2 e 2. Fe(s)Fe2+ 3. Pb2+ Fes) (aq) +2 e (aq)+2 e Pb(s) 4. Pb/sPb2+ 2 e (aq) 2. Use a table of Standard Electron Reduction Potentials (click the Reference Materials link in the header of this webpage and then the Standard Reduction Potentials link) to determine the E° for the following standard cells. Write the net ionic equation for the cell. Identify which half-cell will be the cathode. Directions For Writing Molecular, lonic, And Net lonic Equations: 1. Molecular Equation: balance the molecular equation by predicting the products and changing the coefficients so that all atoms are balanced. Equations must be balanced to obey the Law of Conservation of Matter 2. lonic Equation: the ionic equation is obtained by breaking apart into ions any strong acid, strong base or soluble salt that has an (ag) after its formula - the ion's new coefficient will equal the coefficient (from the balanced equation) TIMES the ion's subscript (i.e. 2 Na2SO4 becomes 4 Na* and 2 SO42-). 3. Do Not Break Apart any reactant or product that has an (s) or () or (g) after its formula - list it exactly as it appears in the molecular equation 4. Do Not Break Apart a weak acid or base that has an (aq) after its formula - by definition a weak acid (or base) is not 100% ionized so it is represented as a molecule and not as ions. 5. Net lonic Equation: write the net ionic equation by cancelling out any spectator ions (ions that appear on both sides of the equation) and simplifying the coefficients to the smallest whole number ratio (if necessary). 6. Make sure to include the charge and state for the ions and molecules - all molecules have a charge of zero. 7.As a final check, the total charge on the reactant side must equal the total charge on the product side - this fact will aid you in writing Red Ox (Reduction Oxidation) reactions.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Solve question b for 1,2 & 3. See instruction

Transcribed Image Text:b. A cell constructed with a Fe | Fe2 half-cell and a Pb | Pb2+ half cell
1. Determine Ecell (V)
2. Write the net ionic equation for the cell.
Work Problem
3. Identify the half-cell at the cathode (enter a number 1 - 4)
1. Fe2+
(aq)+2 e
2. Fe(s)Fe2+
3. Pb2+
Fes)
(aq) +2 e
(aq)+2 e Pb(s)
4. Pb/sPb2+
2 e
(aq)
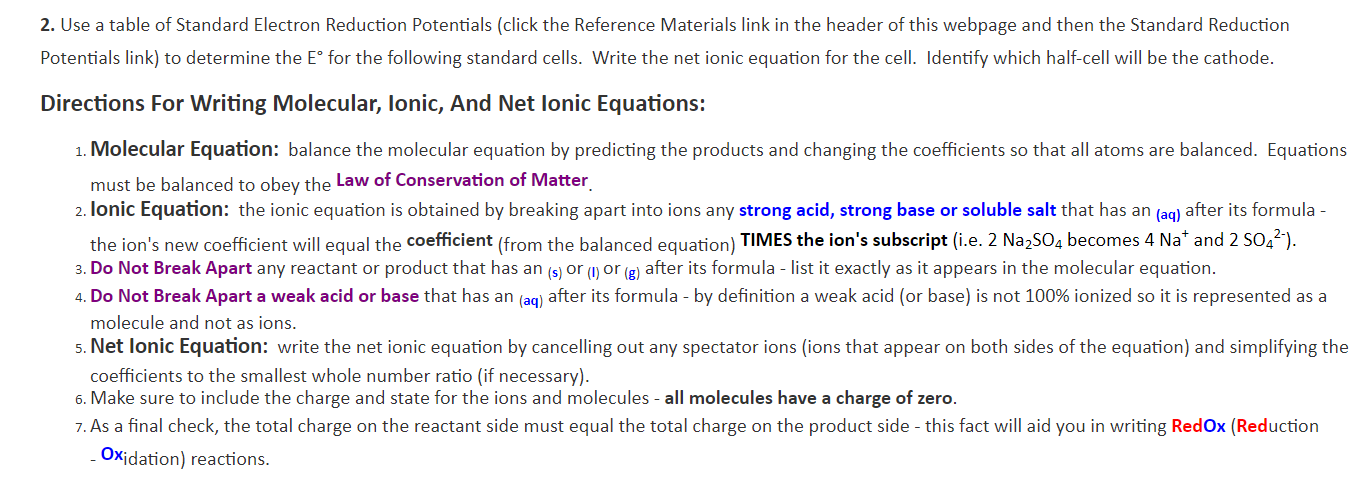
Transcribed Image Text:2. Use a table of Standard Electron Reduction Potentials (click the Reference Materials link in the header of this webpage and then the Standard Reduction
Potentials link) to determine the E° for the following standard cells. Write the net ionic equation for the cell. Identify which half-cell will be the cathode.
Directions For Writing Molecular, lonic, And Net lonic Equations:
1. Molecular Equation: balance the molecular equation by predicting the products and changing the coefficients so that all atoms are balanced. Equations
must be balanced to obey the Law of Conservation of Matter
2. lonic Equation: the ionic equation is obtained by breaking apart into ions any strong acid, strong base or soluble salt that has an (ag) after its formula -
the ion's new coefficient will equal the coefficient (from the balanced equation) TIMES the ion's subscript (i.e. 2 Na2SO4 becomes 4 Na* and 2 SO42-).
3. Do Not Break Apart any reactant or product that has an (s) or () or (g) after its formula - list it exactly as it appears in the molecular equation
4. Do Not Break Apart a weak acid or base that has an (aq) after its formula - by definition a weak acid (or base) is not 100% ionized so it is represented as a
molecule and not as ions.
5. Net lonic Equation: write the net ionic equation by cancelling out any spectator ions (ions that appear on both sides of the equation) and simplifying the
coefficients to the smallest whole number ratio (if necessary).
6. Make sure to include the charge and state for the ions and molecules - all molecules have a charge of zero.
7.As a final check, the total charge on the reactant side must equal the total charge on the product side - this fact will aid you in writing Red Ox (Reduction
Oxidation) reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning