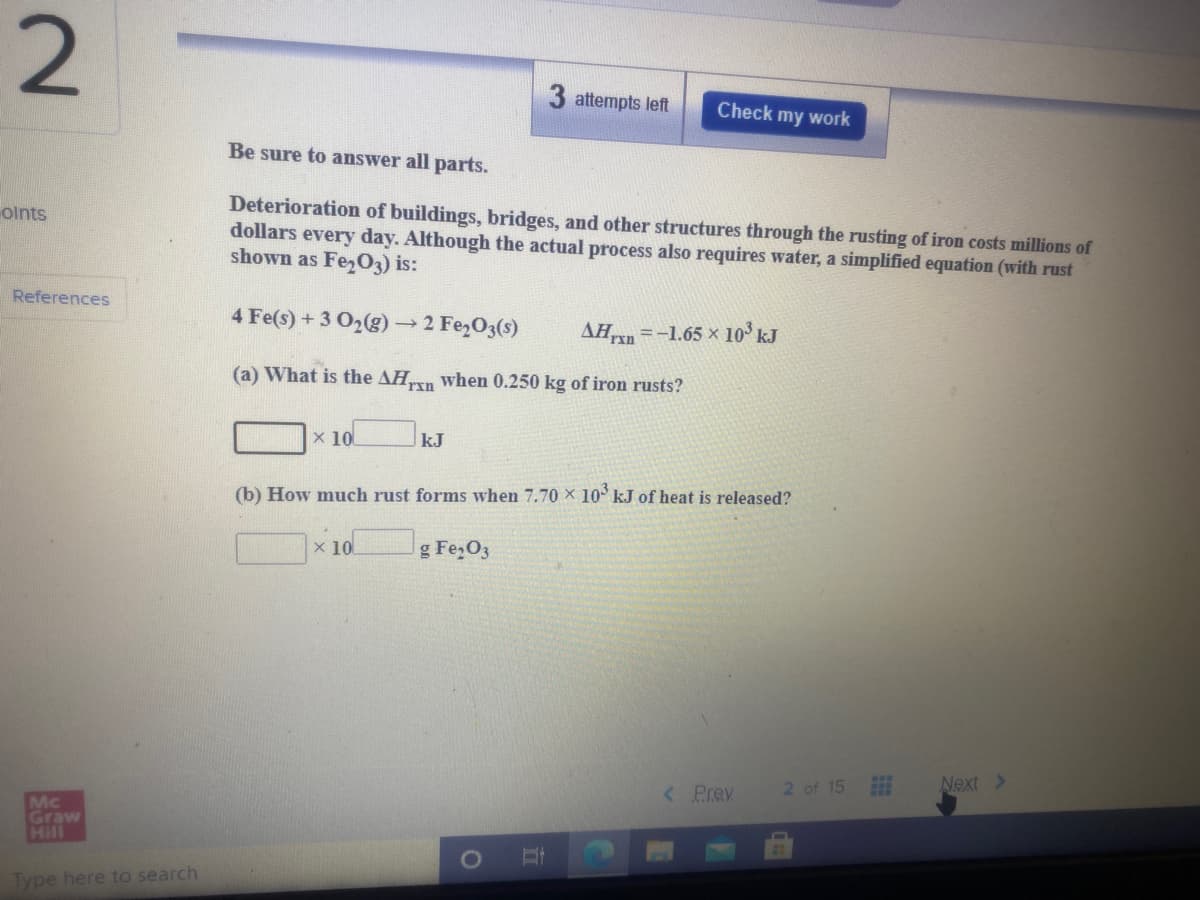
Be sure to answer all parts. Deterioration of buildings, bridges, and other structures through the rusting of iron costs millions of dollars every day. Although the actual process also requires water, a simplified equation (with rust shown as Fe,03) is: 4 Fe(s) +3 O2(g) 2 Fe,O3(s) AHn =-1.65 x 10° kJ (a) What is the AHn when 0.250 kg of iron rusts? x 10 kJ (b) How much rust forms when 7.70 x 10° kJ of heat is released? 1x10 g Fe,O3
Be sure to answer all parts. Deterioration of buildings, bridges, and other structures through the rusting of iron costs millions of dollars every day. Although the actual process also requires water, a simplified equation (with rust shown as Fe,03) is: 4 Fe(s) +3 O2(g) 2 Fe,O3(s) AHn =-1.65 x 10° kJ (a) What is the AHn when 0.250 kg of iron rusts? x 10 kJ (b) How much rust forms when 7.70 x 10° kJ of heat is released? 1x10 g Fe,O3
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.159QP: The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by...
Related questions
Question
Transcribed Image Text:2
3 attempts left
Check my work
Be sure to answer all parts.
Deterioration of buildings, bridges, and other structures through the rusting of iron costs millions of
dollars every day. Although the actual process also requires water, a simplified equation (with rust
shown as FezO3) is:
olnts
References
4 Fe(s) + 3 O2(g)→2 Fe2O3(s)
AĦn =-1.65 x 10° kJ
(a) What is the AHn when 0.250 kg of iron rusts?
x 10
kJ
(b) How much rust forms when 7.70 x 10 kJ of heat is released?
x 10
g Fe,O3
< Prev
2 of 15
Next >
Mc
Graw
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning