Be sure to answer all parts. Predict the product of the following reaction and then draw a stepwise mechanism for its formation: Part 1 out of 3 HBr OH draw structure Next part 10 of 11 Next> < Prev PriSc C F5 F12 F11 F10 F9 F8 F7 F6 F3 & % 0 9 7 4 5 R E T 9LO பி LLI
Be sure to answer all parts. Predict the product of the following reaction and then draw a stepwise mechanism for its formation: Part 1 out of 3 HBr OH draw structure Next part 10 of 11 Next> < Prev PriSc C F5 F12 F11 F10 F9 F8 F7 F6 F3 & % 0 9 7 4 5 R E T 9LO பி LLI
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter8: Addition Via Carbocation
Section: Chapter Questions
Problem 21CTQ
Related questions
Question
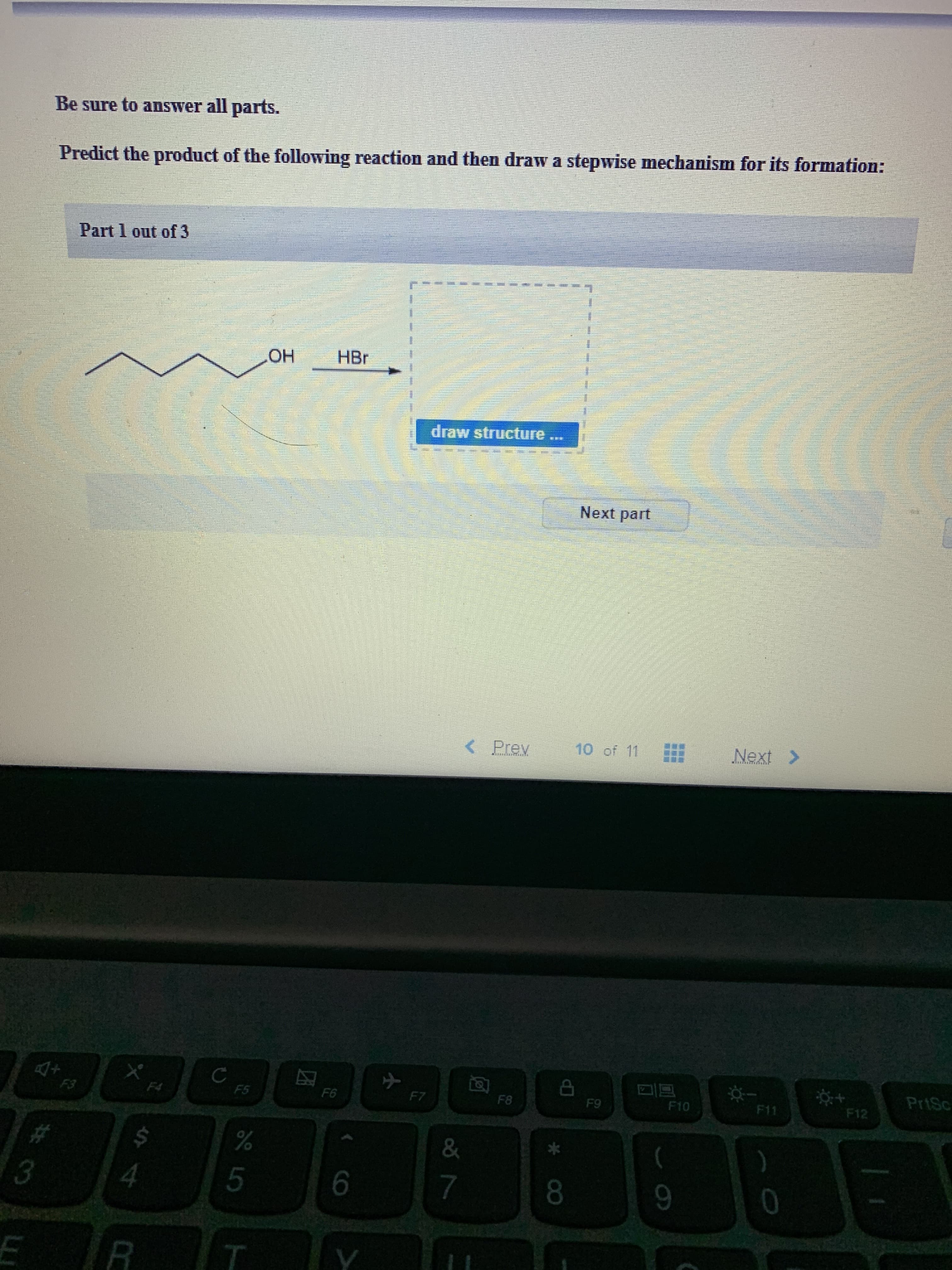
Transcribed Image Text:Be sure to answer all parts.
Predict the product of the following reaction and then draw a stepwise mechanism for its formation:
Part 1 out of 3
HBr
OH
draw structure
Next part
10 of 11
Next>
< Prev
PriSc
C
F5
F12
F11
F10
F9
F8
F7
F6
F3
&
%
0
9
7
4
5
R
E
T
9LO
பி
LLI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1
VIEWTrending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
