Below is a table showing the concentrations over time for a chemical reaction where chemical A turns into chemical B. Time (min) Concentration Concentration A % B % 1 75 25 50 50 40 60 25 .75 1 Co
Below is a table showing the concentrations over time for a chemical reaction where chemical A turns into chemical B. Time (min) Concentration Concentration A % B % 1 75 25 50 50 40 60 25 .75 1 Co
Calculus: Early Transcendentals
8th Edition
ISBN:9781285741550
Author:James Stewart
Publisher:James Stewart
Chapter1: Functions And Models
Section: Chapter Questions
Problem 1RCC: (a) What is a function? What are its domain and range? (b) What is the graph of a function? (c) How...
Related questions
Question
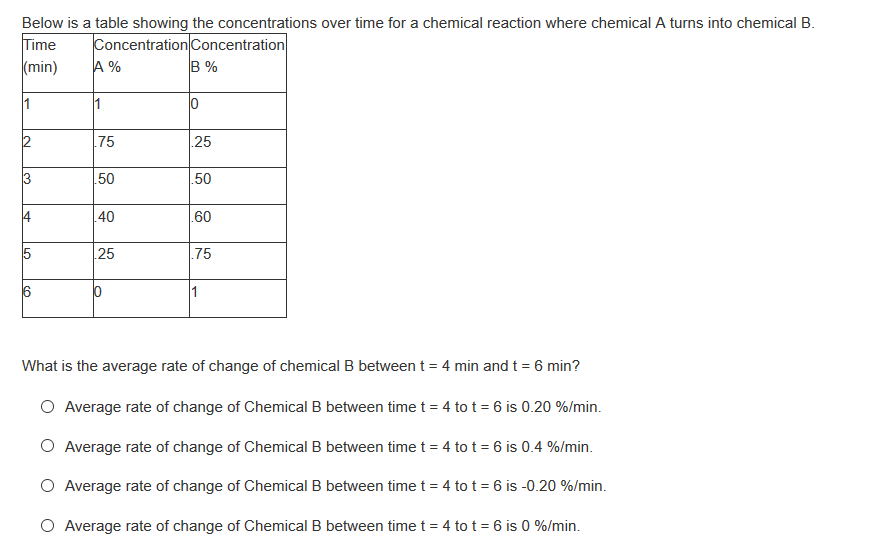
Transcribed Image Text:Below is a table showing the concentrations over time for a chemical reaction where chemical A turns into chemical B.
Time
(min)
Concentration Concentration
A %
B%
1
1
75
25
50
50
4
40
.60
25
.75
6
1
What is the average rate of change of chemical B between t = 4 min and t = 6 min?
O Average rate of change of Chemical B between time t = 4 to t = 6 is 0.20 %/min.
O Average rate of change of Chemical B between time t = 4 to t = 6 is 0.4 %/min.
O Average rate of change of Chemical B between time t = 4 to t = 6 is -0.20 %/min.
O Average rate of change of Chemical B between time t = 4 to t = 6 is 0 %/min.
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, calculus and related others by exploring similar questions and additional content below.Recommended textbooks for you

Calculus: Early Transcendentals
Calculus
ISBN:
9781285741550
Author:
James Stewart
Publisher:
Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:
9780134438986
Author:
Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:
PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:
9780134763644
Author:
William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:
PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:
9781285741550
Author:
James Stewart
Publisher:
Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:
9780134438986
Author:
Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:
PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:
9780134763644
Author:
William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:
PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:
9781319050740
Author:
Jon Rogawski, Colin Adams, Robert Franzosa
Publisher:
W. H. Freeman


Calculus: Early Transcendental Functions
Calculus
ISBN:
9781337552516
Author:
Ron Larson, Bruce H. Edwards
Publisher:
Cengage Learning