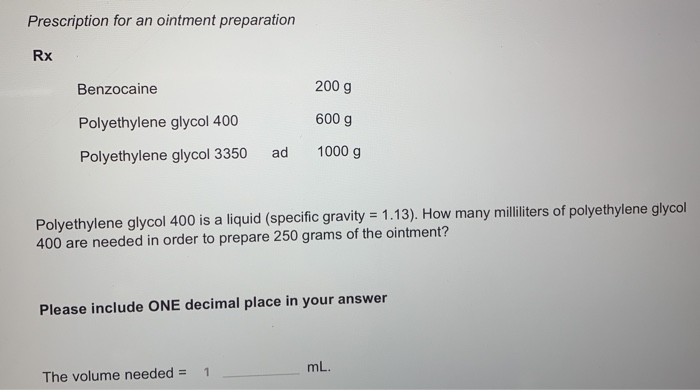
Benzocaine 200 g Polyethylene glycol 400 600 g Polyethylene glycol 3350 ad 1000 g Polyethylene glycol 400 is a liquid (specific gravity = 1.13). How many milliliters of polyethylene glycol 400 are needed in order to prepare 250 grams of the ointment?
Q: Q1: Indicate whether the following statements are true or false ,correct the false and give a proper…
A:
Q: 0.4ml to minims
A: ml and minims are unit of Volume 1 ml = 16.2307 minims 0.4 ml =0.4 × 16.2307 minims0.4 ml =6.49228…
Q: Given the information that the specific gravity of glycerin is 1.25 at 25°C, you will need to add…
A: %w/w is concentration unit to find the mass of solute present in 100 gm of solution we are required…
Q: A prescription requires you to prepare 10 suppositories with 0.3 g of kematopril (density= 3.0 g/mL)…
A: As per the given data, Weight of suppository of cocoa butter = 2.0 g Weight of drug in each…
Q: True or false: Particles with small surface area to volume ratios hold more water than particles…
A: The water holding capacity of particles is important to determine in the field of agriculture as…
Q: A student is asked to prepare 45.0 mg of a sample to be used in a mixed melting point test. The…
A: Given, Mass of the sample needed to be prepared = 45.0 mg % of A in the sample = 65.0 % % of B in…
Q: Calculate the molar concentration of a solution that is 50.0% NaOH (w/w) and has a specific gravity…
A: The molar concentration of a solution that is 50.0% NaOH (w/w) and has a specific gravity of 1.52 is…
Q: If I want to keep a total mass of 17 grams (glycerin) and I have 2 additives of 0.3 grams how much…
A: solutions- This is the addition reaction.
Q: Questions 4 and 5 pertain to the following scenario: The patient needs 500 g of the above ointment…
A: Here we have to determine the mass of betamethasone present in 500 g of ointment having mass…
Q: What is the concentration of the solution prepared by diluting 200 µl of kanamycin (25 mg/ml) to a…
A: (1 )Kanamycin have 25mg/ml That is 1 ml have 25 mg 200 u ml= 10-9x 200 ml = 2 x 10-7 ml have = 2 x…
Q: 4. A. Identify the set-up: B. Function: organic H.O HO HSO OH snoonbe 15. A. Identify the set up: B.…
A: A. The setup shown in figure is separation by funnel. B. FUNCTION Basically this separation…
Q: How many mL of 0.625 M KBr are needed to prepare 2.50L of 0.0500 M KBr? A) 31.3 mL B) 0.0200 mL C)…
A: Which one of the following is correct answer
Q: hardness is 25 mg/L and permanent calcium sulphate hardness 25 5. A sample of water was analyzed and…
A: Hard water is one that does not forms lather with soap. It contains soluble salts of Ca2+ and Mg2+.
Q: The ingredients contained in grape Kool-Aid can be approximated as: water (H2O, density = 1.000…
A: Given: Volume of solution = 500 mL = 0.500 L (Since 1 L = 1000 mL) Mass of…
Q: Calculate the molar concentration of nitric acid (63.0 g/mol) in a solution that has a specific…
A: The relation between molarity (molar concentration), percentage, density and molecular weight is…
Q: Camphor=100g Starch =600g Zinc oxide=300g Question 1: Reduce the formulation to make 30 grams. Show…
A: The composition of the formulation is given in terms of its components as: Camphor, C = 100 g…
Q: A 0.390-mLmL sample of very-low-density lipoprotein (VLDL) has a mass of 371 mgmg. What is the…
A: We have given that Mass of (VLDL) = 371mg=371×10-3grams = 0.371g Volume of (VLDL) = 0.390ml…
Q: Insulin is a protein that is used by the body to regulate both carbohydrate and fat metabolism. A…
A: Given : Volume of insulin = 125ml Concentration = 50mg/ml
Q: 4 mL of a ground water sample from Gallup, NM was found to contain 50 micrograms of U4+ ion. Express…
A: Parts per million (ppm) :- The number of parts by weight of solute present in million (106) parts…
Q: If fifty glycerin suppositories are made from the following formula, how many milliliters of…
A: Solution Glycerol conjointly referred to as glycerin or glycerin) may be a easy polyol compound.…
Q: The formula for lactose is C12H22O11, and the monohydrate is C12H22O11*H2O. What is the percent by…
A: Since you have posted multiple unrelated questions, we are entitled to answer the first only.
Q: The N and P recommendation for a sorghum crop is 140 lbs N/ac and 40 lbs P205/ac. How much urea…
A: Nitrogen and Phosphorus are two of the major components which must be present in a fertilizer.
Q: what is the weight in grams of 300 ml of alcohol with a specific gravity of 0.80
A: Specific density may be defined as the ratio of density of substance with the density of…
Q: If the concentration of a fluoride in a city is drinking water were 0.800 per parts per million how…
A: Given : The concentration of fluoride in parts per million i.e ppm = 0.800 ppm Since there is no…
Q: The volume dispensed from the buret amounts to 32 33 32.25 ml O 33.75 mL O 33.70 ml O 32.20 mL
A: Given,
Q: If you are requested to compound 60 mL of the Concentrated Peppermint Water from the original amount…
A: 500 mL Concentrated Peppermint water contains:- 10 mL of Peppermint Oil + 25 g talc + 300 mL of…
Q: Estimate how much the salinity of the surface water of the Arctic Ocean would need to increase…
A: Density is defined as the mass of water per unit volume and has units of grams per cubic centimeter…
Q: 2. Explain the difference between (a) nanoscience and nanotechnology (b) nanomaterial and…
A: Hey, since there are multiple questions posted, we will answer first question. If you want any…
Q: How can you prepare 500 ml of 0.1M HCI, if the percentage is 35%, and density = 1.18g/ml,…
A: Mass percentage or percentage by weight is one of the concentration terms that indicates the mass of…
Q: Compounds used in pharmacology experiments are often initially made as stock solutions. Stock…
A: Solution - According to the question - Given - 1) Acetylcholine stock concentration is 10mM…
Q: 345. Indicate latin name, raw material and family of Sumac: A) Rhus semialata, cortex Rhus…
A: The given compound = Sumac Sumac is a small tree which grows in north America, east Asia and…
Q: In 1993 the Minnesota Department of Health set a health risk limit for 1,2-dlbromoethane In…
A: Given information, Health risk limit of 1,2-dibromoethane is 4.00 ng/L. Volume of water sample…
Q: Scientists at the University of Idaho Parma Research and Extension Center in Parma, Idaho have…
A: Given: Amount of starch has produce = 2.50×103 kg percent of starch in potato = 1.83 % let 1.83 kg…
Q: Data Set Exp.5 Use the following data to determine the mass percent of the active ingredient in…
A: Calibration curve It is the standard curve used to determine the concentration of unknown sample…
Q: The ingredients contained in grape Kool-Aid can be approximated as: • water (H2O, density = 1.000…
A: Given: Volume of solution = 500 mL = 0.500 L (Since 1 L = 1000 mL) Mass of…
Q: a-) Compound X is steam distilled at 92C under the pressure of 0.977 atm. Amount of water in…
A: a) Given, total pressure of system (X + water) =0.977 atm =742.52 mm Hg vapour pressure of water…
Q: How many milliliters of H2SO4, 94.0% w/w, with a specific gravity of 1.831, are required to prepare…
A:
Q: he water in a dam has been analyzed to contain 0.35 ppb of Selenium. How many micrograms of Selenium…
A: We have to find out the mass of selenium persent in 10L of water
Q: Test tube KI H₂SO4 H₂O2 Volume / cm³ Na2S2O3 Distilled Total Starch solution water
A: Given table is:
Q: If you purchase 1 bottle of 120 tablets of 650. mg sodium bicarbonate in the pharmacy aisle (the…
A:
Q: In the production of printed circuit boards for the electronics industry, a 0.320 mm layer of copper…
A: First the volume of Cu is calculated which then used to determine the mass of Cu.
Q: Consider the following prescription: Promethazine HCl 62.5 mg NaCl Nacl sb 50 mL SWFI ad Make…
A: Given, Promethazine HCl = 62.5 mg NaCl = qs SWFI ad = 50 mL Make isotonic solution E-value of…
Q: A cellulose sponge, 7.0 cm x 12 cm x 2.5 cm, weighs 12.0 grams. Calculate the specific gravity of…
A:
Q: Draw the products of the attached reactions:
A: Lithium diisopropylamine (LDA) is a bulky base that can abstract the α proton from the ketone. At…
Q: Pls compute hwo to prepare the solutions 1. 500.0 mL 0.5 M H2SO4 solution 2. Starch solution a.…
A: We will prepare H2SO4 from the concentrated solution we are having. We should always mix acid with…
Q: 0 30 5060 70 3600 UALA Weight percent tungsten 90 80 100 3422°C 3400 - 3200 3000 2800 2665 C 2670 C…
A: Few questions based on phase diagram that are to be accomplished.
Q: Use the following atomic masses (in g/mol): K = 39.1; Mn = 54.94; O = 16; H = 1; P = 30.97; C =…
A: Given acid-base reaction is H2SO4 + 2 NaOH --> Na2SO4 + 2 H2O At equivalents point Na…
Q: What is the molar concentration of HNO3 (63.0 g/mol) in a solution that has a specific gravity of…
A:
Q: 12. table sugar 13. grain alcohol 14.rubbing alcohol Isopropyl alcohol 15.nail polish C12H22011…
A: The solution of the question is given below:
Q: If fifty glycerin suppositories are made from the following formula, how many milliliters of…
A:
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

- The formula for 1000 g of polyethylene glycol ointment calls for 600 g polyethylene glycol 400. At $ 19.15 per pint, what is the cost of the polyethylene glycol 400, specific gravity 1.140 , needed to prepare 4000 g of ointment?If a pharmacist combined 50-mL portions of three syrups having specific gravities of 1.10, 1.25, and 1.32, what would be the specific gravity (to two decimal places) of the combined product? A laboratory utilizes a mixture of 10% dimethyl sulfoxide (DMSO) in the freezing and long-term storage of embryonic stem cells. If DMSO has a specific gravity of 1.1004, calculate the specific gravity, to four decimal places, of the mixture (assume water to be the 90% portion).The alcohol content of hard liquor is normally given in terms of the “proof,” which is defined as twice the percentage by volume of ethanol (C2H5OH) present. Calculate the number of grams of alcohol present in 1.00 L of 75-proof gin. The density of ethanol is 0.798 g/mL. Round off to 4 sig. figures for all calculations made prior to final answer and the final answer should be in 3 sig. figures
- A 1 mL microbial suspension comparable to 1 mL of 1.0 McFarland standard was subjected to a series of dilutions. An aliquot of 0.2 mL was transferred into a 100-mL volumetric flask and diluted with sterile saline solution to its final volume. Then a 0.1 mL of the resulting solution was transferred into a 50-mL volumetric flask diluted to its final volume. What would be the final concentration?How many microliters of 0.200 M Fe(NO3)3 do you need to make 200 microliters of a 2.50 mM solution?How many microliters of 0.200 M Fe(NO3)3 do you need to make 200 microliters of a 2.50 mM solution?Which option is not a recommended technique for dispensing liquid from a micropipette into another solution? Select one: Place the tip slightly below the level of the solution before dispensing. Dispense the liquid along the inside of the glassware. Submerge the micropipette tip completely into the solution before dispensing. Hold the micropipette at a 45 degree angle when dispensing.
- what is the specific gravity of a substance that displaeces 1.2ml and has a mass of 5.021gA 0.390-mLmL sample of very-low-density lipoprotein (VLDL) has a mass of 371 mgmg. What is the density of the VLDL? Express your answer to three significant figuresEstimate how much the salinity of the surface water of the Arctic Ocean would need to increase before the surface density would equal the potential density at 1000-m depth. how does this compare with the average salinity of the ocean? (Good values might be T=-1.5˚C, S = 32.8, and potential density about 24.5.).
- Seoyeon needs to prepare a 250 mL solution of 0.1000 M Na2CO3. Which glassware should she use to prepare the solution? 250-mL volumetric flask 250-mL graduated cylinder 250-mL Erlenmeyer flask 250-mL beaker For the given data set: [6.7, 7.3, 7.5, 7.2, 7.9, and 7.1] Determine the outlier using Grubbs test. Gcrit = 1.887 6.7 7.9 7.3 7.1 There is no outlier. The results of an analysis for a certain sample are given (in ppm): 20.1, 24.1, 23.5, 22.2, and 21.6. Calculate the 95% confidence interval. 20.3 ppm ≤ x̄ ≤ 24.3 ppm 20.5 ppm ≤ x̄ ≤ 24.1 ppm 20.8 ppm ≤ x̄ ≤ 24.8 ppm 21.0 ppm ≤ x̄ ≤ 24.6 ppmFiltration of samples A, B, and C show no residue left. After letting them dry in different beakers, SAmple A remains no residues. Sample B shows crystal leftovers, and sample C show brown film on the beaker walls. This means that: a. Two or more samples are heterogenous b. Two or more samples are homogenous c. all sample have 2 or more phases d. all samples are pureA student dissolves a Jefferson nickel to make 100.00 mL of solution in a volumetric flask. The student takes a 5.00 mL5.00 mL aliquot of the first solution and dilutes it to make 100.00 mL100.00 mL of a second solution. The student places a sample of the second solution in a cuvette for analysis using spectrophotometry. The molarity of the copper solution in the cuvette was determined by spectrophotometric analysis to be 2.90×10−2 M Cu.2.90×10−2 M Cu. Calculate the mass of copper in the Jefferson nickel used to make the first solution.

